
Sterility Testing Market Share, Size, Trends, Analysis, Industry Report
By Product & Service (Services, Kits & Reagents, Instruments), By Test Type, By Application, By End-Use, By Region and Forecast, 2024 – 2032
- Published Date:Jan-2024
- Pages: 118
- Format: PDF
- Report ID: PM3280
- Base Year: 2023
- Historical Data: 2019-2022
Report Outlook
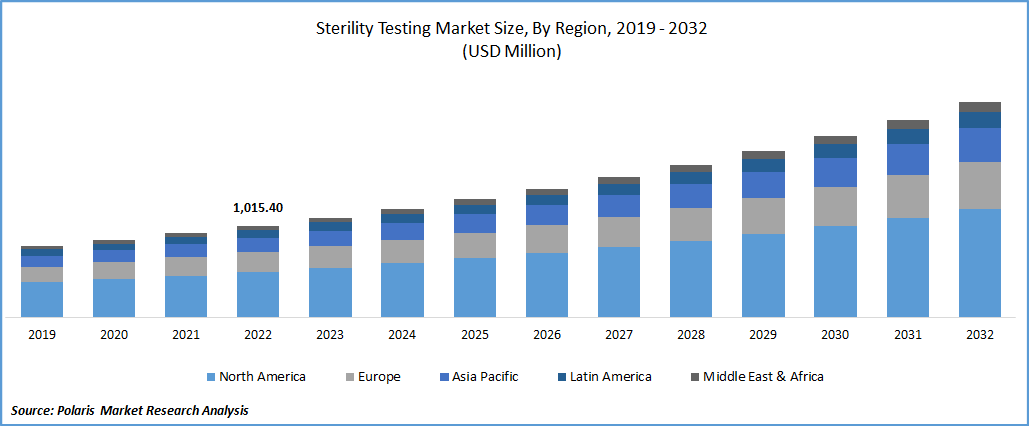
The sterility testing market was valued at USD 1,104.65 million in 2023 and is expected to grow at a CAGR of 9% during the forecast period. Sterility testing is a critical process in the pharmaceutical and medical device industries to ensure the absence of viable microorganisms in a product or sample. It is a crucial component of quality control procedures and helps to ensure the safety and efficacy of a product. The testing is carried out in accordance with regulatory guidelines and involves various techniques to detect the presence of microorganisms.
To Understand More About this Research: Request a Free Sample Report
The sterility testing is to confirm that a product is free from viable microorganisms. This is achieved by exposing the product to culture media under conditions that promote the growth of microorganisms. If any microorganisms are present, they will grow and produce visible colonies on the culture media. The culture media used in sterility testing is designed to support the growth of a broad range of microorganisms, including bacteria, fungi, and yeasts.
Sterility testing can be carried out using different methods, including direct inoculation, membrane filtration, and container closure integrity testing. Direct inoculation involves adding the product directly to culture media and observing the media for any signs of microbial growth. Membrane filtration, on the other hand, involves passing a sample through a filter that retains microorganisms. The filter is then transferred to culture media to promote growth. Container closure integrity testing is a non-destructive method that evaluates the integrity of the product packaging and ensures that it prevents the entry of microorganisms.
Sterility testing is a challenging process and requires careful planning and execution to ensure accurate results. Factors such as sample size, growth conditions, and incubation time can all impact the test outcome. Additionally, the choice of culture media is critical to ensure the detection of a broad range of microorganisms.
sterility testing is a crucial component of quality control procedures in the pharmaceutical and medical device industries. It helps to ensure the safety and efficacy of products by confirming the absence of viable microorganisms. The testing is carried out using various methods, including direct inoculation, membrane filtration, and container closure integrity testing. However, careful planning and execution are essential to ensure accurate results.
Industry Dynamics
Growth Drivers
The global sterility testing market is growing rapidly, driven by various factors such as the increasing demand for biopharmaceuticals, advancements in technology, and growing regulatory requirements. In this response, we will discuss the growth drivers for the sterility testing market in more detail.
The sterility testing market is the increasing demand for biopharmaceuticals. Biopharmaceuticals are becoming increasingly important in the healthcare industry as they offer targeted and effective treatments for a range of diseases. As the demand for biopharmaceuticals grows, so does the need for rigorous testing to ensure their safety and efficacy. Sterility testing is a critical component of the quality control process for biopharmaceuticals, making it an essential market driver.
the sterility testing market. Rapid technological advancements have enabled the development of more efficient and accurate testing methods. For instance, the use of automated systems for sterility testing has increased, which has improved the speed and accuracy of testing while reducing the risk of human error. Additionally, advancements in analytical techniques, such as chromatography and mass spectrometry, have enabled the detection of lower levels of microorganisms, making sterility testing more sensitive.
The growing regulatory requirements for sterility testing are also driving market growth. Regulatory bodies such as the US FDA and the European Medicines Agency (EMA) require sterility testing for various products, including pharmaceuticals, medical devices, and biologics. These requirements ensure that products are safe and effective for use, which is essential for protecting public health. As regulatory requirements become more stringent, the demand for sterility testing will continue to increase.
the sterility testing market is the increasing adoption of quality assurance programs by pharmaceutical and medical device manufacturers. Quality assurance programs ensure that products meet strict quality standards, including sterility testing requirements. The adoption of these programs has increased due to the growing emphasis on patient safety and the need to comply with regulatory requirements. This has led to an increased demand for sterility testing services and products.
Report Segmentation
The market is primarily segmented based on product & service, test type, application, end-use, and region.
|
By Product & Service |
By Test Type |
By Application |
By End-Use |
By Region |
|
|
|
|
|
For Specific Research Requirements: Request for Customized Report
The Kits & Reagents Segment is Expected to Witness the Fastest Growth During the Forecast Period
The kits and reagents segment of the sterility testing market is expected to witness the fastest growth in the coming years. Kits and reagents are essential components of the sterility testing process and are used for sample preparation, culture media preparation, and microbial identification. The growing demand for efficient and reliable testing methods is driving the growth of this segment. The increasing adoption of automated testing systems is one of the key factors driving the growth of the kits and reagents segment. Automated systems provide faster and more accurate results, which are essential for quality control in the pharmaceutical and medical device industries. Additionally, automated systems require specific kits and reagents for use, leading to an increased demand for these products. The growing demand for biopharmaceuticals is also driving the growth of the kits and reagents segment. Biopharmaceuticals are highly complex products that require extensive testing to ensure their safety and efficacy. Sterility testing is a critical component of the quality control process for biopharmaceuticals, and the growing demand for these products is leading to an increased demand for testing kits and reagents.
Advancements in technology are also driving the growth of the kits and reagents segment. New and improved kits and reagents are being developed to meet the growing demand for more efficient and accurate testing methods. For instance, rapid microbial detection kits are being developed that provide faster results than traditional culture-based methods.
The Membrane Filtration Segment Accounted for the Largest Market Share in 2022
The membrane filtration segment accounted for the largest market share in the sterility testing market. Membrane filtration is a widely used method for the detection of microorganisms in pharmaceutical and medical device products. This method involves filtering a liquid or gas sample through a membrane filter with a pore size small enough to trap microorganisms. The filter is then transferred onto a culture media, and the microorganisms are allowed to grow, allowing for their detection and identification.
The widespread use of membrane filtration in the pharmaceutical and medical device industries is one of the key factors driving the growth of this segment. Membrane filtration is a reliable and efficient method for detecting microorganisms, and it is widely accepted by regulatory bodies such as the US FDA and the European Medicines Agency (EMA) as a standard method for sterility testing.
The increasing demand for biopharmaceuticals is also driving the growth of the membrane filtration segment. Biopharmaceuticals are highly complex products that require extensive testing to ensure their safety and efficacy. Sterility testing is a critical component of the quality control process for biopharmaceuticals, and membrane filtration is a widely accepted method for detecting microorganisms in these products.
The growing emphasis on patient safety and the need to comply with regulatory requirements is another factor driving the growth of the membrane filtration segment. Regulatory bodies such as the US FDA and the EMA require sterility testing for various products, including pharmaceuticals, medical devices, and biologics. The use of membrane filtration for sterility testing is widely accepted by these regulatory bodies, which is driving the growth of this segment.
The Demand in North America is Expected to Witness Significant Growth During the Forecast Period
The demand for sterility testing in North America is expected to witness significant growth in the coming years. North America is a significant market for the healthcare industry, with a large and mature pharmaceutical and medical device industry. The growing emphasis on patient safety and compliance with regulatory requirements is driving the demand for efficient and reliable testing methods, such as sterility testing.
The increasing demand for biopharmaceuticals is also driving the growth of the sterility testing market in North America. Biopharmaceuticals are highly complex products that require extensive testing to ensure their safety and efficacy. Sterility testing is a critical component of the quality control process for biopharmaceuticals, and the growing demand for these products is leading to an increased demand for sterility testing.
The growing number of regulatory requirements for sterility testing is another factor driving the growth of the market in North America. Regulatory bodies such as the US FDA and the Canadian Food Inspection Agency (CFIA) require sterility testing for various products, including pharmaceuticals, medical devices, and biologics. The increasing regulatory requirements are leading to an increased demand for sterility testing services in North America.
Competitive Insight
Some of the major players operating in the global market include Charles River Laboratories International, Inc., Merck KGaA, SGS SA, Thermo Fisher Scientific, Inc., Eurofins Scientific, WuXi AppTec, Nelson Laboratories, LLC, Microbac Laboratories, Inc., Pace Analytical Services, LLC, Toxikon Corporation.
Recent Developments
- In June 2022, STEMart unveiled a new offering of microbiology and sterility testing services for medical devices that are non-pyrogenic and sterile.
- In June 2022, Berkshire Sterile Manufacturing inaugurated a new sterility testing isolator that enables the company to perform on-site sterility testing of its GMP batches. The company specializes in the sterile filling of injectable medications, mainly for clinical trials or low commercial demand.
Sterility Testing Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2024 |
USD 1,202.08 million |
|
Revenue Forecast in 2032 |
USD 2,387.31 million |
|
CAGR |
9% from 2024 - 2032 |
|
Base year |
2023 |
|
Historical data |
2019 - 2022 |
|
Forecast period |
2024 - 2032 |
|
Quantitative units |
Revenue in USD million and CAGR from 2024 to 2032 |
|
Segments Covered |
By Product & Service, By Test Type, By Application, By End-Use, By Region |
|
Regional scope |
North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
|
Key Companies |
Charles River Laboratories International, Inc., Merck KGaA, SGS SA, Thermo Fisher Scientific, Inc., Eurofins Scientific, WuXi AppTec, Nelson Laboratories, LLC, Microbac Laboratories, Inc., Pace Analytical Services, LLC, Toxikon Corporation, Becton, Dickinson and Company, Cytovance Biologics, Inc., Lonza Group AG, Novo Nordisk A/S, Sigma-Aldrich Corporation, STERIS plc, Veeva Systems Inc., Cytiva, MilliporeSigma, Pall Corporation. |
FAQ's
key companies in sterility testing market are Charles River Laboratories International, Inc., Merck KGaA, SGS SA, Thermo Fisher Scientific, Inc., Eurofins Scientific, WuXi AppTec, Nelson Laboratories, LLC.
The sterility testing market expected to grow at a CAGR of 8.9% during the forecast period.
The sterility testing market report covering key segments are product & service, test type, application, end-use and region.
key driving factors in sterility testing market are rising adoption of quality assurance programs by pharmaceutical.
The global sterility testing market size is expected to reach USD 2,387.31 million by 2032.
