
Global Onychomycosis Market Size, Share, Trends, Industry Analysis Report: By Type, By Treatment (Topical, Oral and Others), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East & Africa) – Market Forecast, 2024 - 2032.
- Published Date:Aug-2024
- Pages: 115
- Format: PDF
- Report ID: PM5011
- Base Year: 2023
- Historical Data: 2019-2022
Report Outlook
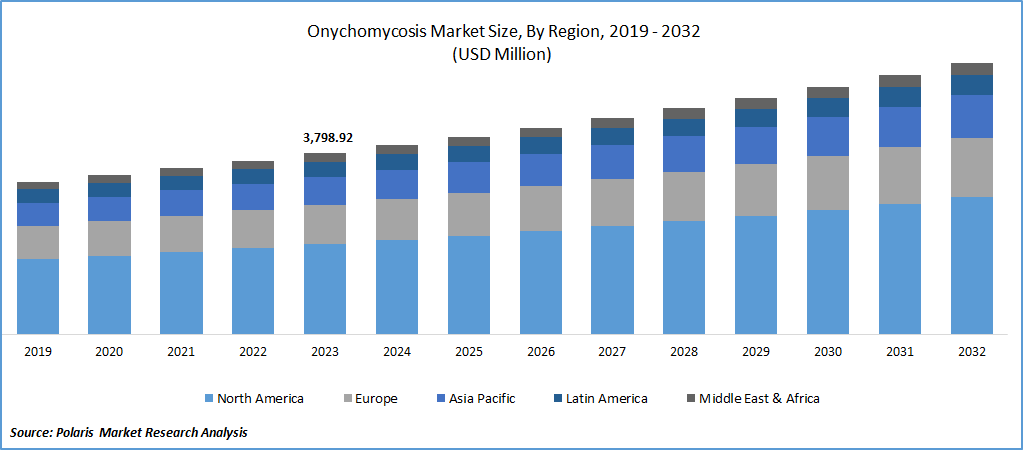
Global onychomycosis market size was valued at USD 3,798.92 million in 2023. The onychomycosis industry is projected to grow from USD 3,968.74 million in 2024 to USD 5,689.46 million by 2032, exhibiting a compound annual growth rate (CAGR) of 4.6% during the forecast period.
The onychomycosis market pertains to treatments and products addressing fungal infections of the nails, affecting millions worldwide, with antifungal medications and topical solutions as primary remedies. The onychomycosis market is experiencing robust growth driven by increased approvals and regulations impacting product approvals, market expansion, and innovation. The companies adhere to regulatory standards to safeguard patient well-being and foster innovation and patient compliance.
Moreover, various stakeholders, including corporations and academic institutions, are advancing innovative and efficient treatments by implementing a range of strategies, such as conducting research and development for onychomycosis therapies to enhance effectiveness and safety, thus boosting onychomycosis market growth.
The growing geriatric population worldwide is anticipated to boost the need for onychomycosis treatment. This is because an individual’s immune system tends to weaken gradually with age, making them more prone to infections like onychomycosis.
To Understand More About this Research:Request a Free Sample Report
For instance, according to the World Health Organization (WHO), by 2030, one out of every six individuals globally will be over the age of 60. The number of individuals above 60 amounted to 1 billion in 2020 and is projected to rise to 1.4 billion in 2030. The elderly population is anticipated to experience the most rapid growth in Eastern and South-Eastern Asia, with a twofold increase expected in the 65 and older age group, followed by Central and Southern Asia, reaching 328.1 million by 2050.
Onychomycosis Market Trends:
Rising Demand for Topical Therapies is Driving the Market Growth
The market growth rate for onychomycosis is being driven by the increasing demand for topical treatments preferred by patients. Such treatments are recommended by doctors based on various factors such as nail involvement, infecting organism, patient characteristics, comorbidities, current medications, biomechanics, as well as cost and accessibility. These factors are taken into consideration in order to create personalized treatment plans for each patient.
The main goal of topical treatment is to eliminate the fungal infection by achieving a mycological cure and restoring the nails to a normal, healthy appearance. Efinaconazole is the preferred initial topical therapy for patients with milder forms of the disease. For older patients with diabetes and severe disease, a combination of topical medications with terbinafine or fluconazole is advised for the topical treatment.
For instance, in July 2022, Zydus received USFDA approval for Efinaconazole Topical Solution, 10%. The medication will be produced at the topical manufacturing facility owned by Zydus Group in India.
Rising Technological Advancements is Driving the Market Growth
The onychomycosis market is experiencing significant growth. The market growth is driven by advanced technologies such as lasers, which are considered effective treatment choices for onychomycosis.
The majority of lasers operate on the concept of selective photothermolysis, in which laser energy is primarily absorbed by the fungal mycelia. This causes a quick rise in temperature within the mycelia, leading to the death of fungal cells. Since the treatment is focused, the neighboring tissue remains unaffected. This reduces the risk of systemic side effects and drives the onychomycosis market revenue.
For instance, Optima Foot and Ankle in North America utilizes the Lunula Laser, the non-thermal laser with FDA 510(k) clearance. Lunula is a dual-diode, Class-2 laser designed to eradicate fungal infections and encourage the growth of healthy new nails. One laser diode utilizes a 405nm wavelength to eliminate the fungus beneath the toenail. The second laser diode operates at a 635nm wavelength to stimulate the germinal/matrix tissue for the production of new, healthy nails.
Onychomycosis Market Segment Insights:
Onychomycosis Type Insights:
The global onychomycosis market segmentation, based on type, includes distal subungual onychomycosis, white superficial onychomycosis, proximal subungual onychomycosis, and others. The distal subungual onychomycosis segment dominated the market owing to the rising number of approvals by the FDA in response to the increasing demand for more effective medications for onychomycosis treatment.
For instance, according to the National Library of Medicine, Terbinafine 10% solution (MOB-015) was granted approval by the U.S. FDA for phase three clinical trials. Terbinafine 10% solution (MOB-015) is an effective choice for treating mild to moderate distal subungual onychomycosis.
Onychomycosis Treatment Insights:
The global onychomycosis market segmentation, based on treatment, includes topical, oral, and others. The topical segment dominated the market over the forecast period as topical treatments are associated with fewer side effects. Moreover, the ongoing research is focused on developing new topical formulations that act more effectively as alternatives to existing topical therapies.
For instance, in January 2024, Vanda Pharmaceuticals received FDA approval to advance with the Investigational New Drug VTR-297, a topical antifungal solution intended for the treatment of onychomycosis.
Global Onychomycosis Market, Segmental Coverage, 2019 - 2032 (USD million)
Source: Secondary Research, Primary Research, PMR Database and Analyst Review
Onychomycosis Regional Insights:
By region, the study provides market insights into North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa. The North America region dominated the market in 2023 owing to the increased occurrence of the target disease and the presence of supportive government policies.
For instance, according to a publication in October 2021 by the American Academy of Family Physicians, the onychomycosis prevalence in North America among adults was estimated to be 13.8% higher than the previous year. This high prevalence is driving the need for approvals from the U.S. Food and Drug Administration (FDA) for effective treatments in managing onychomycosis disease.
For instance, in March 2022, Lupin Limited was granted authorization by the U.S. FDA to market its abbreviated new drug application (ANDA), Efinaconazole Topical Solution, 10%, in the American market. The product will be manufactured at Lupin's facility in India.
Further, the major countries studied in the market report are the US, Canada, Germany, France, the UK, Italy, Spain, China, Japan, India, Australia, South Korea, and Brazil.
The U.S. onychomycosis market accounted for the largest market share in the North American region, owing to the rising prevalence of onychomycosis disease and the growing awareness of the significant potential of onychomycosis drugs in the treatment of this disease.
For instance, according to a report published in 2020 by the National Institute of Health, onychomycosis, a fungal infection that mainly affects the nail, nail bed, and surrounding tissues, is a highly prevalent infection and impacts around 32 million people in the United States.
This rise in onychomycosis has led American companies to allocate substantial funds to acquire and merge with other key companies to contribute towards the advancement of these medications.
The Asia-Pacific onychomycosis market is expected to grow at the fastest CAGR from 2024 to 2032, owing to the high prevalence of onychomycosis in the region. Several factors contribute to the rising prevalence of onychomycosis, including limited access to healthcare services, a growing elderly population, poor hygiene practices, and extensive urbanization. The humid and warm climate in the Asia-Pacific region further exacerbates the prevalence of onychomycosis. Furthermore, major industry players are concentrating on developing innovative products to drive the Asia Pacific onychomycosis market revenue.
For instance, in March 2021, the Tianjin Institute of Pharmaceutical Research and HUYABIO International partnered to file a new drug application (NDA) for antifungal drug efinaconazole, as Jublia, to treat onychomycosis.
Global Onychomycosis Market, Regional Coverage, 2019 - 2032 (USD million)
Source: Secondary Research, Primary Research, PMR Database and Analyst Review
Onychomycosis Key Market Players & Competitive Insights
Leading market players are investing heavily in research and development in order to expand their product lines, which will help the onychomycosis market grow even more. Market participants are also undertaking a variety of strategic activities to expand their global footprint, with important market developments including new product launches, contractual agreements, mergers and acquisitions, higher investments, and collaboration with other organizations. To expand and survive in a more competitive and rising market environment, the onychomycosis industry must offer cost-effective items.
Manufacturing locally to minimize operational costs is one of the key business tactics used by manufacturers in the global onychomycosis industry to benefit clients and increase the market sector. In recent years, the onychomycosis industry has witnessed some technological advancements. Major players in the onychomycosis market include Bausch Health Companies Inc., Moberg Pharma AB, Abbott, Pfizer Inc., Teva Pharmaceutical Industries Ltd., Cipla Inc., Merck & Co., Inc., Novartis AG, Zydus Lifesciences and Sun Pharmaceutical Industries Ltd.
Zydus Lifesciences Limited develops and manufactures pharmaceuticals, including generic drugs, biosimilars, vaccines, APIs, formulations, wellness, and animal health products. Zydus has a global presence, with manufacturing sites and research facilities spread across five states in India, the US and Brazil. The company’s products are marketed in regulated markets like the US, Europe, Latin America, and South Africa, as well as 25 other emerging markets worldwide. In July 2022, Zydus Lifesciences was granted final approval by the United States Food and Drug Administration (USFDA) for the sale of Efinaconazole Topical Solution, 10% across the globe for the treatment of onychomycosis.
Moberg Pharma AB is a pharmaceutical company that specializes in developing and manufacturing topical treatments for skin conditions. The company's product portfolio includes Emtrix/Nalox for nail fungus and psoriasis, Kerasal for dry and damaged feet, and Kaprolac for various skin and scalp problems. The company has a global presence, with operations in over 35 countries, including the US, Europe, Latin America, and South Africa. Moberg Pharma is headquartered in Sweden, Europe. In August 2023, Moberg Pharma AB declared that MOB-015 had been granted national approval specifically for the treatment of mild to moderate onychomycosis in adult patients for the company's domestic market in Sweden.
Key companies in the onychomycosis market include:
- Abbott
- Bausch Health Companies Inc.
- Cipla Inc.
- Merck & Co., Inc.
- Moberg Pharma AB
- Novartis AG
- Pfizer Inc.
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Zydus Lifesciences
Onychomycosis Industry Developments
- February 2024: Allderma AB introduced the Terclara brand for the sale of MOB-015, effective in the treatment of onychomycosis across the European region.
- December 2021: Bausch Health Companies Inc. revealed that JUBLIA (efinaconazole) Topical Solution, 10%, a remedy for onychomycosis, was awarded the American Podiatric Medical Association (APMA) Seal of Approval as it enhances foot health.
- February 2021: Lupin Limited was granted approval by the US Food and Drug Administration for its Tavaborole Topical Solution indicated for onychomycosis.
Onychomycosis Market Segmentation:
Onychomycosis Type Outlook
- Distal Subungual Onychomycosis
- Proximal Subungual Onychomycosis
- White Superficial Onychomycosis
- Others
Onychomycosis Treatment Outlook
- Topical
- Oral
- Others
Onychomycosis Regional Outlook
- North America
- US
- Canada
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Netherlands
- Russia
- Rest of Europe
- Asia-Pacific
- China
- Japan
- India
- Malaysia
- South Korea
- Indonesia
- Australia
- Vietnam
- Rest of Asia-Pacific
- Middle East & Africa
- Saudi Arabia
- UAE
- Israel
- South Africa
- Rest of Middle East & Africa
- Latin America
- Mexico
- Brazil
- Argentina
- Rest of Latin America
Onychomycosis Report Scope:
|
Report Attributes |
Details |
|
Market Size Value in 2023 |
USD 3,798.92 million |
|
Market Size Value in 2024 |
USD 3,968.74 million |
|
Revenue Forecast in 2032 |
USD 5,689.46 million |
|
CAGR |
4.6% from 2024 – 2032 |
|
Base Year |
2023 |
|
Historical Data |
2019 – 2022 |
|
Forecast Period |
2024 – 2032 |
|
Quantitative Units |
Revenue in USD million and CAGR from 2024 to 2032 |
|
Report Coverage |
Revenue Forecast, Market Competitive Landscape, Growth Factors, and Trends |
|
Segments Covered |
|
|
Regional Scope |
|
|
Competitive Landscape |
|
|
Report Format |
|
|
Customization |
Report customization as per your requirements with respect to countries, region, and segmentation. |
FAQ's
The global onychomycosis market size was valued at USD 3,798.92 million in 2023 and is projected to be valued at USD 5,689.46 million in 2032.
The global market is projected to grow at a CAGR of 4.6% during the forecast period, 2024-2032
North America held the largest share of the global market.
The key players in the market are Bausch Health Companies Inc., Moberg Pharma AB, Abbott, Pfizer Inc., Teva Pharmaceutical Industries Ltd., Cipla Inc., Merck & Co., Inc., Novartis AG, Zydus Lifesciences, and Sun Pharmaceutical Industries Ltd.
The distal subungual onychomycosis category dominated the market in 2023
The topical category held the largest share of the global market
