
Leprosy Treatment Market Share, Size, Trends, Industry Analysis Report, By Drug Class (Antibacterial, Leprostatics, Sulfone, Phenazine, Anti-tubercular Drugs, and Others); By Disease Type; By Route of Administration; By Distribution Channel; By Region; Segment Forecast, 2023 - 2032
- Published Date:Jul-2023
- Pages: 112
- Format: PDF
- Report ID: PM3635
- Base Year: 2022
- Historical Data: 2019-2021
Report Outlook
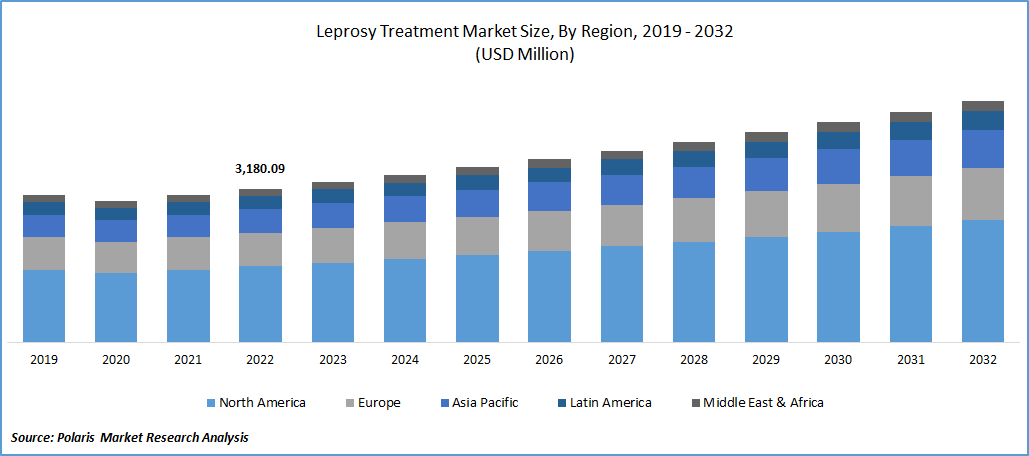
The global Leprosy Treatment market was valued at USD 3,180.09 million in 2022 and is expected to grow at a CAGR of 4.6% during the forecast period.
Hansen's disease, also known as leprosy, is a chronic infection caused by the Mycobacterium leprae bacteria. Symptoms, including skin, eyes, respiratory tract, and nerve granulomas, may go unnoticed for five to twenty years. Anyone, regardless of age, can contract leprosy. Delayed diagnosis can lead to complications such as muscle spasms, erectile dysfunction, organ failure, and vision loss. To prevent leprosy, it is best to avoid contact with untreated individuals. If left untreated, leprosy can cause loss of extremity tissue and other health problems. However, leprosy is treatable with medications such as multidrug therapy (MDT), which involves taking a combination of drugs recommended by the World Health Organisation. Government initiatives and reimbursement policies have helped fuel the market for leprosy treatment. Leprosy patients have been impacted by COVID-19, with non-urgent hospital consultations and admissions being discouraged. This has led to a shortage of hospital resources and increased community-level satellite clinics. However, there have also been positive impacts, including the discovery that the anti-leprosy drug Clofazimine exhibits strong antiviral activities against SARS-CoV-2, which causes COVID-19.
To Understand More About this Research: Request a Free Sample Report
Industry Dynamics
Growth Drivers
As per the National Institute of Health, a neglected tropical disease called fascioliasis, also known as liver fluke infestation, affects 2.4 million people worldwide and poses a risk of infection to an additional 180 million. It is brought on by two parasitic flatworms, infecting people after larvae are consumed in tainted water or food.
Key market players are concentrating on various business strategies, such as obtaining product approval from regulatory authorities, to diversify their product offerings and gain a competitive edge. In February 2019, the US Food and Drug Administration (FDA) authorized Egaten to treat fascioliasis in six or older patients. This makes Egaten the only medication for those with this condition that has received FDA approval, and it should make it easier for people to access it not just in the US but also in other countries that are affected by it. These approvals have bolstered the Leprosy Treatment market growth.
Furthermore, expanding government initiatives and favourable reimbursement policies fuel the market for leprosy treatment. The Indian government started the National Leprosy Control Programme (NLCP) in 1954–1955. The National Leprosy Eradication Programme was introduced in 1983, and Multi-Drug Therapy (MDT) began to be widely used in 1982. The NLEP's strategy was based on breaking the chain of disease transmission by reducing the population's overall infection rate and the number of infectious sources. However, it is anticipated that the high cost of medications and the general lack of awareness will restrain the market's expansion for leprosy treatments.
For Specific Research Requirements, Request for a Customized Report
Report Segmentation
The market is primarily segmented based on drug class, route of administration, distribution channel, disease type, and region.
|
By Drug Class |
By Disease Type |
By Route of Administration |
By Distribution Channel |
By Region |
|
|
|
|
|
To Understand the Scope of this Report: Speak to Analyst
The Multibacillary Leprosy Segment is Expected to Witness the Fastest Growth During Forecast Period
The segment is growing at a faster rate due to it providing treatment to leprosy patients rapidly. When discussing drug regimens in the United States, only the terms paucibacillary and multibacillary are typically used. Patients with paucibacillary disease have a negative skin smear and a negative biopsy for more advanced disease. Patients with multibacillary disease are those whose skin smears or biopsies show a more severe form of the disease. Rifampicin and dapsone are used to treat PB leprosy for six months, while rifampicin, dapsone, and clofazimine are used to treat MB leprosy for twelve months.
Antibacterial Drugs Accounted for Significant Market Share in 2022
Doctors frequently prescribe antibacterial medications to treat leprosy; as a result, these medications are anticipated to hold a significant market share in 2022 and beyond. Antibacterial drugs have a low relapse rate, resistance, and side effects, fueling industry growth. The most popular antibacterial medications are rifampin, dapsone, ofloxacin, clofazimine, and minocycline, also suggested by the WHO for leprosy multidrug therapy. While many medicines are available for patients with late-stage leprosy, early diagnosis is crucial for effective treatment.
The Demand in North America is Expected to Witness Significant Growth During Forecast Period
A significant portion of the global market is anticipated to be accounted for by North America because of the region's favorable reimbursement environment, high healthcare spending, and quick adoption of new technologies. Hansen's disease is a rare condition in the US. Hansen's disease causes up to 2 million people to be permanently disabled worldwide. There is very little chance that any adult worldwide will contract Hansen's disease. This is because over 95% of people have built-in immunity to the illness.
During the forecast period, Europe is anticipated to trail North America in market share for leprosy treatments due to the region's residents' high disposable income and growing awareness of new treatment options. During the forecast period, rapid growth is anticipated in the Asia Pacific region. Leprosy prevalence is rising, government initiatives are increasing, and the region's healthcare infrastructure is developing quickly, all of which can be linked to market expansion.
Competitive Insight
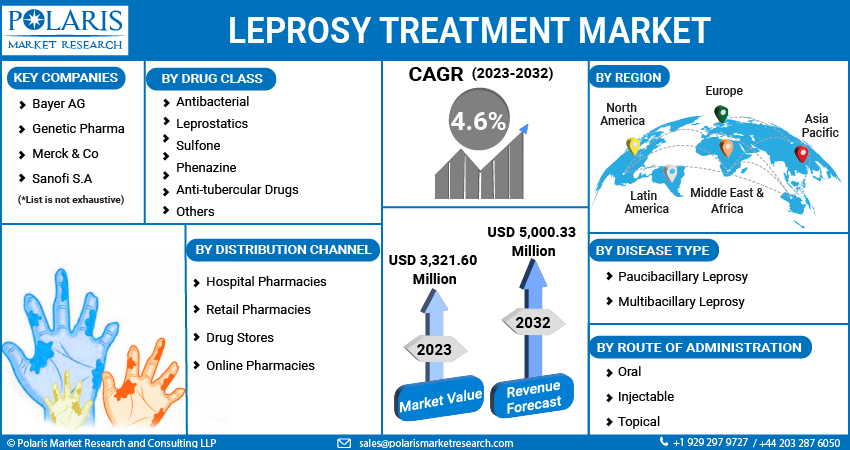
Some of the major players operating in the global market include Acme Pharmaceuticals, Astra Zeneca, Bayer, Bristol-Myers, Cadila Pharmaceuticals, Eli Lily, Genetic Pharma, GSK, IDPL, Johnson & Johnson, Lark Laboratories, Merck, Macleods Pharmaceuticals, Novartis, Pfizer, Systopic Laboratories, Sanofi, & Teva Pharmaceutical.
Recent Developments
In February 2021, The World Health Organisation (WHO) and Novartis renewed their partnership, which will fuel the global effort to eradicate leprosy. Novartis will continue to donate multi-drug therapy (MDT) medications to treat leprosy through the end of 2025, Owing to the five-year extension of the partnership, which was first established in 2000.
Leprosy Treatment Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2023 |
USD 3,321.60 million |
|
Revenue Forecast in 2032 |
USD 5,000.33 million |
|
CAGR |
4.6% from 2022 - 2030 |
|
Base year |
2022 |
|
Historical data |
2019 - 2021 |
|
Forecast period |
2023 - 2032 |
|
Quantitative units |
Revenue in USD million/billion and CAGR from 2023 to 2032 |
|
Segments Covered |
By Drug Class, By Disease Type, By Route of Administration, By Distribution Channel, By Region |
|
Regional scope |
North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
|
Key Companies |
Acme Pharmaceuticals, Astra Zeneca Pharma India Ltd., Bayer AG, Bristol-Myers Squibb and Company, Cadila Pharmaceuticals Corp., Eli Lily and Company, Genetic Pharma, Glaxo Smithcline Pharmaceuticals Ltd., IDPL, Johnson & Johnson Services Inc., Lark Laboratories (India) Ltd., Merck & Co., Macleods Pharmaceuticals, Novartis International AG, Pfizer Inc., Systopic Laboratories Pvt. Ltd., Sanofi S.A., and Teva Pharmaceutical Industries Ltd. |
FAQ's
key companies in Leprosy Treatment Market are Acme Pharmaceuticals, Astra Zeneca, Bayer, Bristol-Myers, Cadila Pharmaceuticals, Eli Lily, Genetic Pharma.
The global Leprosy Treatment market expected to grow at a CAGR of 4.6% during the forecast period.
The Leprosy Treatment Market report covering key are drug class, route of administration, distribution channel, disease type, and region.
key driving factors in Leprosy Treatment Market are Increasing product approval from regulatory authorities.
The global Leprosy Treatment market size is expected to reach USD 5,000.33 million by 2032.
