
Latent TB Testing Market Size, Share, Trends, Industry Analysis Report: By Test Type (IGRA and TST), Application, End User, and Region (North America, Europe, Asia Pacific, Latin America, and Middle East & Africa) – Market Forecast, 2025–2034
- Published Date:Apr-2025
- Pages: 129
- Format: PDF
- Report ID: PM5494
- Base Year: 2024
- Historical Data: 2020-2023
Latent TB Testing Market Overview
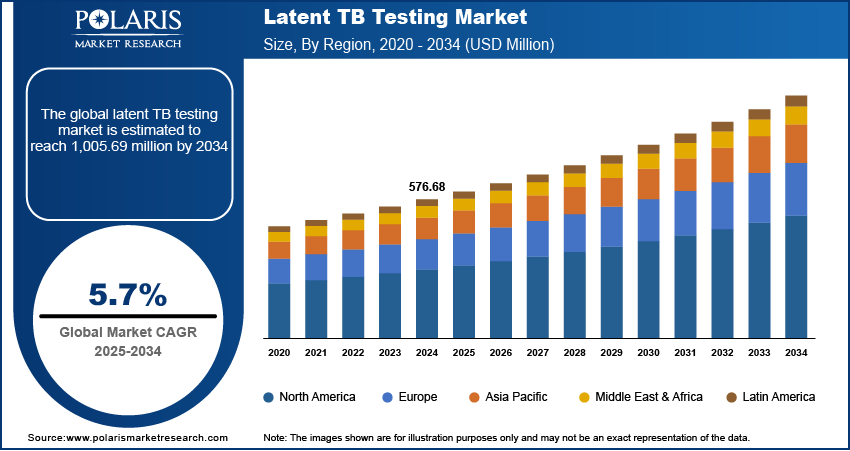
The global latent TB testing market size was valued at USD 576.68 million in 2024. It is expected to grow from USD 608.95 million in 2025 to USD 1,005.69 million by 2034, at a CAGR of 5.7% during 2025–2034.
Latent TB testing refers to diagnostic methods used to detect Mycobacterium tuberculosis infection in individuals who do not exhibit active symptoms but are at risk of developing tuberculosis. The latent TB testing market growth is driven by ongoing technological advancements that improve diagnostic accuracy and efficiency. Innovations such as interferon-gamma release assays (IGRAs) and next-generation molecular diagnostics offer improved sensitivity, reducing false positives and minimizing the need for follow-up testing. Additionally, automation and digital integration in testing processes streamline laboratory workflows, supporting large-scale screening programs. In February 2025, Qure.ai’s AI-enabled qXR software is being used for TB surveillance by analyzing chest X-rays. It flagged 36.22% as abnormal, with 12% showing presumptive TB signs, aiding early detection and proactive case identification through incidental screening. These advancements are crucial in high-burden regions where early detection plays a substantial role in tuberculosis prevention and control.
To Understand More About this Research: Request a Free Sample Report
Increasing awareness and education initiatives accelerate the adoption of latent TB testing by improving public and healthcare professionals’ understanding of its importance. For instance, in December 2024, India’s NTEP launched a 100-day campaign to accelerate TB elimination, focusing on high-burden districts. Critical strategies include intensive screening, early diagnosis, nutritional support, and community engagement to raise awareness, reduce stigma, and improve treatment adherence for high-risk populations. Government-led campaigns, nonprofit organizations, and healthcare agencies emphasize early diagnosis to prevent disease progression, reducing the global TB burden. Medical training programs and community outreach efforts enhance screening rates, particularly in high-risk populations such as immunocompromised individuals and healthcare workers. Furthermore, collaborations between public health institutions and diagnostic companies drive accessibility, ensuring that testing solutions reach underserved areas. This growing awareness allows a proactive approach to tuberculosis management, reinforcing the critical role of latent TB testing in global health strategies.
Latent TB Testing Market Dynamics
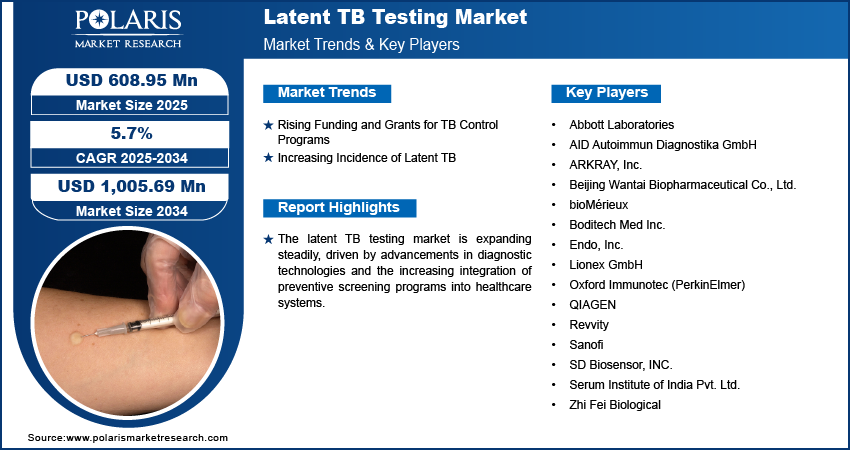
Rising Funding and Grants for TB Control Programs
Government agencies, global health organizations, and private foundations allocate significant financial resources to combat tuberculosis, with a strong focus on early detection and prevention. In September 2024, the Global Fund reported providing 76% of international TB financing, investing USD 9.9 billion in TB prevention and treatment programs, and an additional USD 1.9 billion in TB/HIV initiatives as of June 2024, supporting global efforts to combat tuberculosis. These funds support the procurement of diagnostic kits, the establishment of testing facilities, and the implementation of nationwide screening programs, particularly in high-burden regions. Additionally, increased financial backing encourages research and development efforts, leading to innovations in testing methods that improve accuracy and efficiency. Sustained funding strengthens healthcare infrastructure and expands the reach of latent TB testing, ultimately contributing to better disease management and control. Rising funding and grants for TB control programs boost the latent TB testing market growth.
Increasing Incidence of Latent TB
The rising incidence of latent TB is driving the demand for latent TB diagnostic testing, as a growing number of individuals carry the infection without exhibiting symptoms. For instance, in May 2024, the CDC estimated that up to 13 million people in the US have latent TB infection. Without treatment, 5%–10% of these individuals may develop active TB disease over their lifetimes, highlighting the importance of early detection and intervention. This increase is particularly concerning in regions with high TB prevalence, densely populated urban areas, and among immunocompromised individuals, where the risk of disease progression is elevated. Early detection through latent TB testing is essential for preventing the transition to active tuberculosis, which can have severe public health implications. Healthcare systems are prioritizing routine screening and preventive treatment strategies as the global burden of latent TB rises, further propelling the need for accurate and accessible testing solutions. This growing emphasis on proactive disease management underscores the critical role of latent TB diagnostics in controlling tuberculosis transmission and reducing long-term healthcare costs.
Latent TB Testing Market Segment Insights
Latent TB Testing Market Assessment by Test Type Outlook
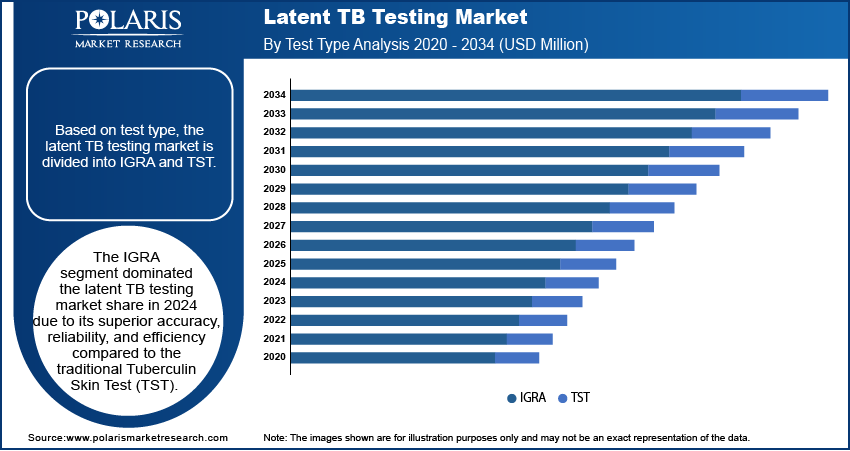
The global latent TB testing market segmentation, based on test type, includes IGRA and TST. The IGRA segment dominated the latent TB testing market share in 2024 due to its superior accuracy, reliability, and efficiency compared to the traditional Tuberculin Skin Test (TST). IGRA eliminates the risk of false positives caused by prior Bacillus Calmette-Guérin (BCG) vaccination, a common limitation of TST, making it the preferred choice in both developed and developing healthcare settings. Additionally, IGRA offers a faster turnaround time and does not require a second patient visit for result interpretation, improving compliance and streamlining diagnostic workflows. These advantages, combined with increasing adoption by healthcare providers and advancements in laboratory automation, have contributed to IGRA's leading position in the market.
Latent TB Testing Market Evaluation by End User Outlook
The global latent TB testing market segmentation, based on end user, includes diagnostic laboratories, hospitals & clinics, academic & research institutes, and others. The hospitals & clinics segment is expected to witness the highest latent TB testing market growth during the forecast period, driven by their role as primary healthcare providers for routine screenings and diagnostic services. Hospitals and clinics have well-established infrastructure, advanced diagnostic capabilities, and access to a larger patient population, making them key contributors to latent TB detection. Additionally, the integration of TB screening into routine check-ups, pre-employment health assessments, and pre-surgical evaluations has fueled demand for testing services in these settings. The presence of specialized healthcare professionals and the ability to provide immediate follow-up care further enhance the efficiency and reliability of TB diagnosis, supporting the rapid growth of this segment.
Latent TB Testing Market Regional Analysis
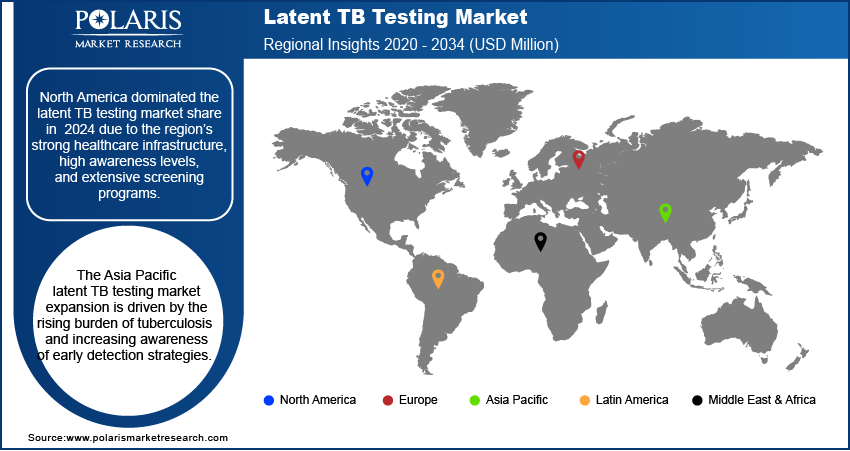
By region, the report provides the latent TB testing market insights into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America dominated the latent TB testing market revenue in 2024 due to the region’s strong healthcare infrastructure, high awareness levels, and extensive screening programs. The presence of advanced diagnostic technologies and the widespread adoption of IGRA-based testing have further strengthened the market in this region. In March 2024, the WHO, in collaboration with FIND and Unitaid, launched a new TB sequencing portal featuring over 56,000 sequences. This platform serves as the most advanced knowledge base for Mycobacterium tuberculosis sequencing and phenotyping, enhancing global TB research and diagnostics. Additionally, government initiatives and public health policies promoting TB prevention and early detection have significantly contributed to latent TB testing market expansion. The high prevalence of latent TB among immigrant populations and immunocompromised individuals, such as those suffering from HIV or undergoing immunosuppressive therapy, has also driven demand for reliable and accurate testing solutions. For instance, in 2022, 72.9% of newly reported TB cases in the US were among non-US born individuals. These factors collectively position North America as a leader in the global latent TB testing market.
The Asia Pacific latent TB testing market is projected to witness the fastest growth during the forecast period, driven by the rising burden of tuberculosis and increasing awareness of early detection strategies. Countries in this region are implementing large-scale screening programs, particularly in high-risk populations, to curb TB transmission. Expanding healthcare infrastructure, government funding, and collaborations with global health organizations are further supporting the accessibility of advanced diagnostic solutions. Additionally, the growing adoption of IGRA-based testing, alongside improvements in laboratory capabilities, is enhancing the efficiency and accuracy of latent TB detection. These developments, coupled with an increasing focus on preventive healthcare, are expected to drive significant latent TB testing market expansion in Asia Pacific.
Latent TB Testing Market Players & Competitive Analysis Report
The competitive landscape features global leaders and regional players competing for latent TB testing market share through innovation, strategic alliances, and regional expansion. Global players leverage strong R&D capabilities, technological advancements, and extensive distribution networks to deliver advanced diagnostic solutions, meeting the growing demand for accurate, efficient, and accessible testing methods. Latent TB testing market trends highlight the rising demand for emerging technologies, digitalization, and automation in diagnostics, driven by increasing TB prevalence, geopolitical shifts, and macroeconomic trends. Global players focus on strategic investments, mergers and acquisitions, and joint ventures to strengthen their market position. Post-merger integration and strategic alliances are key strategies to improve competitive positioning and expand regional footprints. Regional companies, meanwhile, address localized needs by offering cost-effective solutions and leveraging their understanding of regional healthcare landscapes. Competitive benchmarking in the latent TB testing market includes market entry assessments, expansion opportunities, and partnership ecosystems to meet the demand for innovative and future-ready diagnostic solutions. The market is experiencing substantial technological advancements, such as molecular diagnostics, automated testing platforms, and digital health integration, which are reshaping industry ecosystems. Companies are investing in supply chain management, procurement strategies, and sustainability transformations to align with market demand and regulatory requirements. Pricing insights, revenue growth analysis, and competitive intelligence are critical for identifying opportunities and driving long-term profitability. In conclusion, the latent TB testing market opportunities lie in technological innovation, market adaptability, and regional investments. Major players focus on strategic developments, market penetration, and competitive benchmarking to address economic and geopolitical shifts, ensuring sustained growth in a hypercompetitive global market. The increasing focus on early detection improved diagnostic accuracy, and accessibility underscores the importance of advanced latent TB testing solutions in combating the global TB burden. A few key major players are QIAGEN; Revvity; Beijing Wantai Biopharmaceutical Co., Ltd.; Sanofi; Endo, Inc.; bioMérieux; SD Biosensor, INC.; Lionex GmbH; Serum Institute of India Pvt. Ltd.; ARKRAY, Inc.; Zhi Fei Biological; AID Autoimmun Diagnostika GmbH; Boditech Med Inc.; Oxford Immunotec (PerkinElmer); and Abbott Laboratories.
QIAGEN is a global provider of Sample to Insight solutions, offering a range of diagnostic tools, including those for latent tuberculosis (TB) testing. The company's flagship product in this area is the QuantiFERON-TB Gold Plus (QFT-Plus), an Interferon-Gamma Release Assay (IGRA) that provides more reliable and objective results compared to traditional tuberculin skin tests. QFT-Plus is widely recommended by health organizations such as the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC) for identifying individuals at risk of developing active TB from latent infection. This test measures the cell-mediated immune response to specific TB antigens in whole blood, offering high sensitivity and the ability to detect infection at early stages. QIAGEN also offers the LIAISON QuantiFERON-TB Gold Plus test, developed in partnership with DiaSorin, which provides an automated solution for high-throughput detection of latent TB. Additionally, QIAGEN's QIAreach QuantiFERON-TB is a semi-automated option designed for use in conjunction with risk assessment and other diagnostic evaluations. These products play a crucial role in global TB control efforts by accurately identifying latent TB infections, which can progress to active TB if left untreated.
bioMérieux is a global player in the field of in vitro diagnostics, offering innovative solutions for various health challenges, such as tuberculosis (TB). In the context of latent TB testing, bioMérieux has developed the VIDAS TB-IGRA, a fully automated Interferon-Gamma Release Assay (IGRA) designed to aid in the diagnosis of latent TB infection. This assay is performed on the VIDAS 3 immunoanalyzer, providing high sensitivity and specificity for detecting Mycobacterium tuberculosis infection. The VIDAS TB-IGRA is particularly notable for its ease of use, requiring minimal sample preparation and offering quick results, making it an efficient tool for hospital laboratories and private clinics. The launch of VIDAS TB-IGRA aligns with bioMérieux's commitment to improving public health by providing reliable diagnostic tools. This assay is part of the company's broader TB diagnostic portfolio, which also includes solutions for active TB detection. bioMérieux supports global efforts to control TB by identifying and treating latent infections before they progress to active disease by offering a fully automated and reliable method for latent TB testing. The company's focus on automation and traceability improves workflow efficiency, making it easier for healthcare providers to manage TB testing in-house.
List of Key Companies in Latent TB Testing Market
- Abbott Laboratories
- AID Autoimmun Diagnostika GmbH
- ARKRAY, Inc.
- Beijing Wantai Biopharmaceutical Co., Ltd.
- bioMérieux
- Boditech Med Inc.
- Endo, Inc.
- Lionex GmbH
- Oxford Immunotec (PerkinElmer)
- QIAGEN
- Revvity
- Sanofi
- SD Biosensor, INC.
- Serum Institute of India Pvt. Ltd.
- Zhi Fei Biological
Latent TB Testing Industry Development
April 2024: Revvity launched the Auto-Pure 2400 liquid handler to use with the T-SPOT.TB test, enhancing lab efficiency for latent TB testing. The system processes 24 samples per run, simplifies workflows, and maintains high clinical accuracy, benefiting mid-to-high-volume labs.
March 2024: QIAGEN collaborated with the International Panel Physicians Association to promote TB screening awareness, focusing on IGRA testing benefits. This aligns with updated US CDC guidelines mandating IGRA tests for immigrants from high-TB incidence countries, supporting global TB prevention efforts.
Latent TB Testing Market Segmentation
By Test Type Outlook (Volume, Number of Tests in Thousands; Revenue, USD Million, 2020–2034)
- IGRA
- TST
By Application Outlook (Volume, Number of Tests in Thousands; Revenue, USD Million, 2020–2034)
- People Living with HIV
- HHC with TB
- Others
By End User Outlook (Volume, Number of Tests in Thousands; Revenue, USD Million, 2020–2034)
- Diagnostic Laboratories
- Hospitals & Clinics
- Academic & Research Institutes
- Others
By Regional Outlook (Volume, Number of Tests in Thousands; Revenue, USD Million, 2020–2034)
- North America
- US
- Canada
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Netherlands
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Malaysia
- South Korea
- Indonesia
- Australia
- Vietnam
- Rest of Asia Pacific
- Middle East & Africa
- Saudi Arabia
- UAE
- Israel
- South Africa
- Rest of Middle East & Africa
- Latin America
- Mexico
- Brazil
- Argentina
- Rest of Latin America
Latent TB Testing Market Report Scope
|
Report Attributes |
Details |
|
Market Size Value in 2024 |
USD 576.68 million |
|
Market Size Value in 2025 |
USD 608.95 million |
|
Revenue Forecast in 2034 |
USD 1,005.69 million |
|
CAGR |
5.7% from 2025 to 2034 |
|
Base Year |
2024 |
|
Historical Data |
2020–2023 |
|
Forecast Period |
2025–2034 |
|
Quantitative Units |
Volume, Number of Tests in Thousands; Revenue in USD Million and CAGR from 2025 to 2034 |
|
Report Coverage |
Revenue Forecast, Market Competitive Landscape, Growth Factors, and Industry Trends |
|
Segments Covered |
|
|
Regional Scope |
|
|
Competitive Landscape |
|
|
Report Format |
|
|
Customization |
Report customization as per your requirements with respect to countries, regions, and segmentation. |
FAQ's
The global latent TB testing market size was valued at USD 576.68 million in 2024 and is projected to grow to USD 1,005.69 million by 2034.
The global market is projected to register a CAGR of 5.7% during the forecast period.
North America dominated the market revenue in 2024.
A few of the key players in the market are QIAGEN; Revvity; Beijing Wantai Biopharmaceutical Co., Ltd.; Sanofi; Endo, Inc.; bioMérieux; SD Biosensor, INC.; Lionex GmbH; Serum Institute of India Pvt. Ltd.; ARKRAY, Inc.; Zhi Fei Biological; AID Autoimmun Diagnostika GmbH; Boditech Med Inc.; Oxford Immunotec (PerkinElmer); and Abbott Laboratories.
The IGRA segment dominated the market share in 2024.
The hospitals & clinics segment is expected to witness the fastest market growth during the forecast period.
