
Human Platelet Lysate Market Share, Size, Trends, Industry Analysis Report, By Type (Heparin Based HPL, Heparin Free HPL); By Application; By End-Use; By Region; Segment Forecast, 2023 - 2032
- Published Date:Oct-2023
- Pages: 116
- Format: PDF
- Report ID: PM3990
- Base Year: 2022
- Historical Data: 2019-2021
Report Outlook
The global human platelet lysate market was valued at USD 50.76 million in 2022 and is expected to grow at a CAGR of 3.9% during the forecast period.
Human Platelet Lysate (HPL) has emerged as a pivotal component in the field of biomedical research and regenerative medicine, revolutionizing cell culture techniques and therapeutic applications. This versatile product, derived from human blood platelets, offers numerous advantages over traditional cell culture supplements like Fetal Bovine Serum (FBS). The growing demand for HPL can be attributed to several driving factors, including its safety, efficiency, and ethical considerations.
To Understand More About this Research: Request a Free Sample Report
HPL, in essence, is a growth supplement used in cell culture to promote the proliferation and maintenance of various cell types. Its unique composition, rich in growth factors and cytokines, makes it an ideal choice for researchers and clinicians alike. One of the primary driving factors behind the increasing use of HPL is its superior safety profile. Unlike FBS, HPL does not carry the risk of transmitting bovine diseases or causing immunological reactions, making it a safer option for both research and clinical applications. This enhanced safety aspect has garnered widespread recognition and trust within the scientific community.
Parkinson's disease, a progressive neurodegenerative disorder, has prompted extensive research into regenerative therapies that can potentially slow or reverse its debilitating effects. Platelet lysate, with its rich source of growth factors and cytokines, has become a valuable component in these therapies. Researchers are exploring the use of platelet lysate in cell-based treatments to stimulate neuronal regeneration and enhance the quality of life for individuals living with Parkinson's.
- For instance, As of July 25, 2022, data available from the National Institutes of Health (NIH) revealed that around 500,000 individuals receive a diagnosis of Parkinson's disease annually in the United States.
Furthermore, the regulatory landscape has also contributed to the growth of the HPL market. Regulatory bodies in many countries have been advocating for safer and more transparent research practices, which include the use of ethically sourced and safe cell culture supplements. HPL, with its favorable safety and ethical profiles, aligns with these regulatory requirements, easing the approval processes for clinical trials and therapeutic applications.

For Specific Research Requirements: Request for Customized Report
The versatility of HPL extends its applicability to various research areas and clinical applications. It has found extensive use in stem cell research, tissue engineering, and cell therapy development. Stem cell expansion and differentiation, for instance, are greatly facilitated by the growth factors present in HPL, making it an essential component in advancing regenerative medicine. Furthermore, HPL has shown promise in enhancing the production of therapeutic cells, such as mesenchymal stem cells and immune cells, for use in treating various diseases.
The market for HPL is expected to continue its growth trajectory, driven by the convergence of these factors. Researchers and clinicians increasingly recognize the benefits of HPL over traditional cell culture supplements, due to a growing demand for HPL products and services. Biotechnology companies specializing in HPL production are also expanding their capacities to meet this rising demand, contributing to the market's expansion.
Industry Dynamics
Growth Drivers
Increasing In the Research and Development (R&D) Activities of Stem Cell Therapy by Major Companies and Governments
The platelet lysate market has witnessed a significant surge in research and development (R&D) activities, driven by major companies and governments alike. Stem cell therapy, an emerging field with immense potential for regenerative medicine, has been a focal point of this growing interest. Governments around the world have initiated several strategic measures to support and advance R&D efforts in stem cell therapy, consequently influencing the human platelet lysate market.
Governments have recognized the transformative potential of stem cell therapy in addressing a wide range of medical conditions, from degenerative diseases to injuries that were once considered irreversible. Consequently, they have allocated substantial funding and resources to foster innovation and facilitate clinical trials in this domain. By providing financial incentives, research grants, and streamlined regulatory pathways, governments have encouraged both public and private sector stakeholders to invest in the development of stem cell-based treatments.
Additionally, governments have played a crucial role in establishing ethical guidelines and frameworks for stem cell research and therapy. These regulations ensure that research activities are conducted with the highest standards of safety and ethics, which is particularly important given the sensitivity and complexity of stem cell-related work. These regulatory efforts have bolstered public trust in the field and paved the way for responsible advancements.
Report Segmentation
The market is primarily segmented based on type, application, end-use, and region.
|
By Type |
By Application |
By End-Use |
By Region |
|
|
|
|
To Understand the Scope of this Report: Speak to Analyst
By Type Analysis
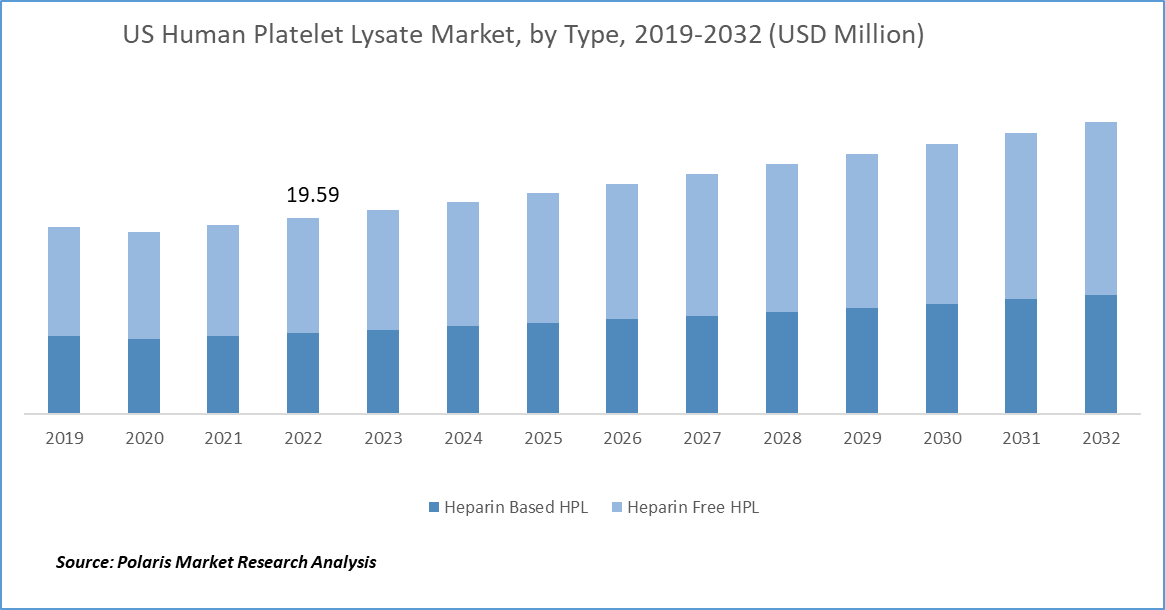
In 2022, the heparin free HPL segment held the largest revenue share
The heparin free HPL segment held the largest revenue share of 58.7% in 2022. It is emerged as a critical component in cell culture and regenerative medicine, offering a serum-free alternative to traditional growth supplements. For instance, Captivate Bio launched PLTGold human platelet lysate that is a heparin-free, xeno-free supplement and is superior alternative to human AB or FBS serum for the growth of MSCs and T cells. This innovative product is gaining traction in the global market, driven by several factors that have reshaped the landscape of cell culture research and therapeutic development.
Human Platelet Lysate, devoid of heparin, represents a breakthrough for researchers and biotechnology companies seeking a safer and more standardized solution for cell expansion and differentiation. Unlike heparin-containing counterparts, heparin-free platelet lysate eliminates potential contaminants and side effects associated with heparin, making it a preferred choice for various applications.
One of the key driving factors behind the increasing demand for heparin-free Human Platelet Lysate is its safety profile. Heparin, a commonly used anticoagulant, can introduce variability and unwanted effects when used in cell culture, affecting cell behavior and complicating downstream analyses. The absence of heparin in platelet lysate mitigates these concerns, offering researchers more reliable and consistent results.
By Application Analysis
The therapeutic segment expected to witness the fastest market during the forecast period
The therapeutic segment expected to witness the fastest market of 4.0% during the forecast period. Human platelet lysate (hPL) has emerged as a promising and versatile therapeutic tool in recent years, revolutionizing the field of regenerative medicine and cell-based therapies. One of the primary uses of hPL is in cell culture and tissue engineering. It serves as a robust alternative to fetal bovine serum (FBS), a commonly used cell culture supplement, particularly for the expansion of mesenchymal stem cells (MSCs). hPL provides essential growth factors such as platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), and vascular endothelial growth factor (VEGF), which support cell proliferation and differentiation.
Furthermore, hPL has its utility in the treatment of various medical conditions, especially those related to tissue repair and regeneration. In the term of wound healing, hPL is topically applied to chronic, non-healing wounds, diabetic ulcers, and burns. The growth factors present in hPL promote angiogenesis, fibroblast proliferation, and extracellular matrix production, accelerating the wound healing process. Additionally, hPL also has application in ophthalmology, where it can be used to treat corneal defects and promote ocular surface reconstruction.
The applications of hPL extend to neurological disorders as well. Recent studies have explored its potential in neurodegenerative diseases like Alzheimer's and Parkinson's disease. The growth factors in hPL may have neuroprotective and neurotrophic effects, which could potentially slow down disease progression and improve neuronal function.
In the field of cancer therapy, hPL presents an interesting paradox. While its growth-promoting factors can be beneficial for regenerating healthy tissues, they may also promote the growth of cancer cells. Therefore, careful consideration is essential when using hPL in cancer patients, and its application should be customized to the specific clinical scenario.
Regional Insights
North America dominated the largest market in 2022
The North America dominated the largest market of 43.5% in 2022, due to primarily used in biomedical research, regenerative medicine, and cell culture. North America is a hub for stem cell research, encompassing pluripotent stem cells, induced pluripotent stem cells (iPSCs), and adult stem cells. HPL is employed to culture and expand these cells for research into developmental processes and therapeutic applications. In the United States, stem cell culture serves as a fundamental element in the realms of regenerative medicine and biomedical research.
Stem cells possess the remarkable capacity to differentiate into diverse cell types and tissues, rendering them invaluable for exploring developmental biology and for potential therapeutic purposes. Oversight of stem cell research and cultivation falls under the purview of federal bodies, including the Food and Drug Administration (FDA) and the National Institutes of Health (NIH). Cell therapy is a dynamic and swiftly advancing field within the United States, offering pioneering treatment avenues for a wide spectrum of medical conditions.
A pivotal aspect of the development and assessment of these therapies is the execution of clinical trials. The United States harnesses a range of cell therapy methodologies, including CAR-T Cell Therapy (the genetic modification of a patient's T cells to selectively target and eliminate cancer cells), Tissue Engineering (the cultivation of replacement tissues or organs in laboratory settings), and Allogeneic and Autologous Cell Therapies (the utilization of cells either from external donors, allogeneic, or from the patients themselves, autologous, for therapeutic purposes). These techniques are instrumental in fostering the growth and advancement of the US market in this domain.
The market in the Asia Pacific region has been experiencing fastest growth in recent years. Platelet lysate is a valuable component in cell culture and regenerative medicine, driving its demand in various research and clinical applications. Increasing prevalence of chronic diseases and the rising demand for regenerative therapies in the Asia Pacific region. As the region grapples with a growing aging population and a higher incidence of degenerative diseases, there is a greater need for innovative treatments, such as stem cell therapies and tissue engineering, which often rely on human platelet lysate as a crucial component.
As the demand for platelet lysate and related products continues to rise in the Asia Pacific region, both international and local companies are actively participating in research and development efforts, ensuring the availability of high-quality platelet lysate products. These key players contribute to advancing scientific research, regenerative therapies, and biotechnology applications across the Asia Pacific region, facilitating innovation and growth in the field. Additionally, the region's improving healthcare infrastructure, coupled with growing investments in research and development, has further propelled the market.
Key Market Players & Competitive Insights
The human platelet lysate market is expected to continue growing as regenerative medicine and stem cell therapies gain prominence in healthcare and research. Competition among key market players will likely intensify, due to ongoing innovation, improved product offerings, and a focus on quality and regulatory compliance.
Some of the major players operating in the global market include:
- AventaCell BioMedical Corp
- Compass Biomedical Inc.
- Lifescience Group Limited
- Macopharma SA
- Merck KGaA
- Mill Creek Lifesciences LLC
- Sclavo Diagnostics International Srl
- Stem Cell Technologies Inc.
- Trinova Biochem GmbH
- Zen Bio, Inc.
Recent Developments
- In June 2023, PL BioScience GmbH entered into a Patent License and Assignment Agreement with French company Macopharma S.A.S., granting PL BioScience a worldwide license under Macopharma's patents and introducing PL BioScience to Macopharma's HPL customers for future orders.
- In June 2021, Captivate Bio has signed a partnership agreement with PL BioScience GmbH to exclusively distribute ELAREM human platelet lysate products in the US and Canada, expanding their HPL offerings and enhancing PL BioScience's market presence in North America.
Human Platelet Lysate Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2023 |
USD 52.74 million |
|
Revenue forecast in 2032 |
USD 74.74 million |
|
CAGR |
3.9% from 2023 – 2032 |
|
Base year |
2022 |
|
Historical data |
2019 – 2021 |
|
Forecast period |
2023 – 2032 |
|
Quantitative units |
Revenue in USD million and CAGR from 2023 to 2032 |
|
Segments Covered |
By Type, By Application, By End-Use, By Region |
|
Regional scope |
North America, Europe, Asia Pacific, Latin America; Middle East & Africa |
|
Customization |
Report customization as per your requirements with respect to countries, region and segmentation. |
FAQ's
key companies in human platelet lysate market are Merck KGaA, Compass Biomedical Inc., AventaCell BioMedical Corp
The global human platelet lysate market is expected to grow at a CAGR of 3.9% during the forecast period.
The human platelet lysate market report covering key segments are type, application, end-use, and region.
key driving factors in industrial human platelet lysate market are 1. Increasing In the Research and Development (R&D) Activities of Stem Cell Therapy by Major Companies and Governments.
The global human platelet lysate market size is expected to reach USD 74.74 million by 2032.
