
HPV Testing and Pap Test Market Share, Size, Trends, Industry Analysis Report, By Test (Pap, HPV); By Application (Cervical Cancer Screening, Vaginal Cancer Screening); By Product (Instruments, Consumables, Services); By Technology (PCR, Immunodiagnostics, 2021 - 2028
- Published Date:Oct-2021
- Pages: 111
- Format: PDF
- Report ID: PM2103
- Base Year: 2020
- Historical Data: 2016 - 2019
Report Outlook
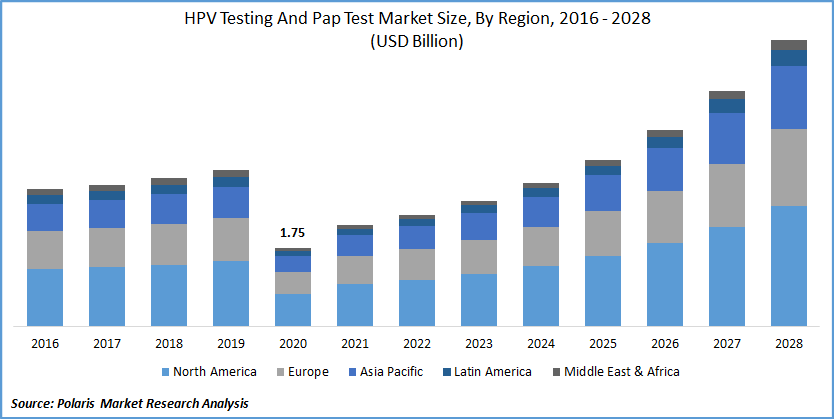
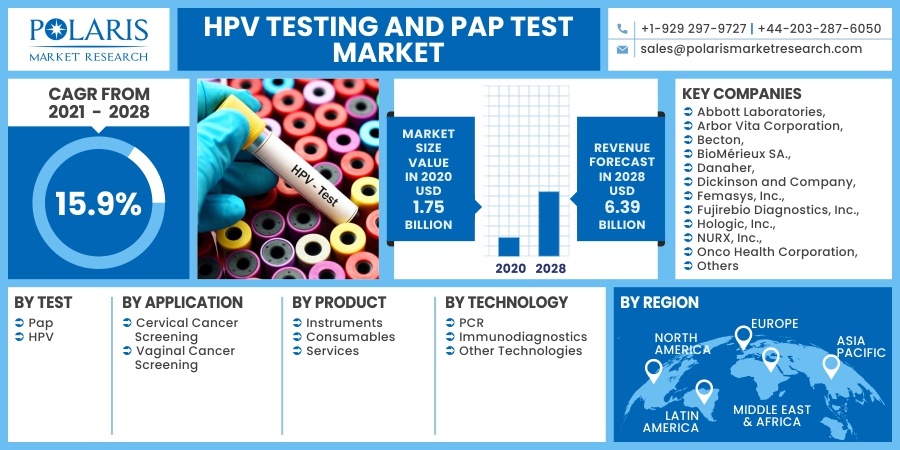
The global HPV testing and Pap test market was valued at USD 1.75 billion in 2020 and is expected to grow at a CAGR of 15.9% during the forecast period. The growing number of cervical cancer cases and cancer screening programs are the primary factors that drive the global market. The upgrading medical and healthcare facility scenario and the rising usage of the newest innovative technologies in medical sciences will further attract significant market revenue over the projected period. Surging knowledge of cancer treatment will further help the market growth in the future years. In addition, the growing assistance from governments around the world in the form of reimbursement policies and healthcare coverage will aid the industry in gaining traction in the near future.
 Know more about this report: request for sample pages
Know more about this report: request for sample pages
Cervical cancer is the fourth common type of cancer affecting women globally. Nearly all cervical cancer cases are related with HPV infection. Hence, HPV testing along with Pap smear testing are common methods for testing to diagnose cervical cancer. The number of cervical cancers is expected to increase to 700,000 cases per year by 2030. This increasing incidence will drive the need for pap testing, which is expected to boost the market growth.
The Hpv Testing And Pap Test Market report details key market dynamics to help industry players align their business strategies with current and future trends. It examines technological advances and breakthroughs in the industry and their impact on the market presence. Furthermore, a detailed regional analysis of the industry at the local, national, and global levels has been provided.
The emergence of the COVID-19 pandemic is expected to hinder the market's growth during the initial phase of the forecast period. Due to the lockdown imposed in many countries, diagnostics testing is being postponed. According to reports, during the first half of 2020, cervical cancer screening was reduced by 94% in the U.S. The pandemic situation has worsened in some countries, and it will take few more months for the situation to stabilize across the globe.
Know more about this report: request for sample pages
Industry Dynamics
Growth Drivers
The global HPV testing and Pap test market growth will be driven by the increasing incidence of cervical cancer cases globally, leading to the increased number of pap examinations globally. The adoption of new innovative technologies in HPV examination and Pap testing is expected to propel the market's growth during the forecast period.
The adoption of new technologies in HPV and PAP testing is expected to significantly contribute to market growth in the coming years. Machine-assisted screening and HPV cell-free DNA methods are being developed for the screening of cervical cancer.
Cell-free DNA is commonly found in cancer patients and is used as a diagnostic tool in various types of cancer. Still, the potential of circulating HPV DNA (HPV cDNA) in cervical cancer screening is not explored. Hence, some researchers are developing a plasma HPV cell-free DNA assay using ddPCR to detect and monitor the treatment response of HPV-associated cancers.
WHO is also adopting various methods such as vaccination, screening, and proper treatment to eliminate cervical cancer by 2050. To fulfill this aim, WHO expects to screen 70% of women aged 35 to 45 years by modern screening pap testing. All these factors will cumulatively contribute to the market growth.
Companies are launching new products and are involved in funding to develop their market outreach. For instance, Roche received FDA approval for Cobas HPV examination using Cobas 6800/8800 Systems in April 2020. NeuMoDx Molecular received European support for NeuMoDx HPV in 2020.
It is an automated real-time PCR assay that runs on NeuMoDx 288 and NeuMoDx 96 molecular systems. Naveris, Inc. also raised USD 19 Million to commercialize the HPV testing based on blood samples in December 2020. The technology detected cancer using circulating tumor DNA and was developed by the University of North Carolina Lineberger Cancer Center.
Report Segmentation
The market is primarily segmented on the basis of test, application, product, technology, and region.
|
By Test |
By Application |
By Product |
By Technology |
By Region |
|
|
|
|
|
Know more about this report: request for sample pages
Insight by Application
The cervical cancer screening segment generated the highest revenue in 2020 and is anticipated to have a high growth rate over the forecast period. This is due to the greater occurrence of cervical cancer than vaginal cancer. Furthermore, the ongoing public-private initiative to raise screening rates is anticipated to boost the segment for pap testing.
The World Health Organization has released the Plan of Action in 2018 for Cervical Cancer Prevention and Control 2018-2030 to improve disease testing and treatment through creative methodologies. The vaginal cancer screening segment is anticipated to have a double-digit CAGR during the forecast timeframe. The growing cases of vaginal cancer and the high mortality rates associated with it have fueled the need for efficient screening tools.
Insight by Product
The consumables segment generated the highest revenue, in 2020, due to its frequent use in HPV and cervical screenings. In addition, ongoing development initiatives by significant market players and the launch of innovative consumables, including assays and kits, for more precise Pap testing and HPV testing, are expected to drive segment growth.
The services segment is predicted to grow throughout the projected timeframe due to the development of at-home and self HPV screening services to boost the number of cervical cancer screening rates in major regions.
Insight by Technology
The other segment dominated the market and generated the highest revenue in 2020. The others segment includes cystoscopy and colposcopy. The increased share can be due to the widespread use of these technologies in HPV testing and PAP testing. PCR testing segment is expected to grow at a high growth rate during the projected period due to the introduction of novel guidelines to use DNA HPV testing methods. This is due to their improved efficiency and cost-effectiveness.
The PAP testing enhances the accuracy of cervical cancer screening programs by detecting dangerous lesions early in women aged 30 and later with normal cytology. It also decreases the requirement for unnecessary colposcopy and therapy in women aged 21 and up who have cytology results showing abnormal squamous cells of unknown significance.
Geographic Overview
North America dominated the HPV testing and Pap test market in 2020 and is expected to remain the same during the projected timeframe. Increasing knowledge regarding early detection of cervical cancer, well-established pap testing protocols, availability of healthcare reimbursement are factors that drive PAP industry growth.
Furthermore, the region's high accessibility, adoption, and availability of technologically sophisticated products and services have expanded the market. Also, the presence of key companies in the region and their ongoing strategic endeavors have aided the market's expansion significantly in the region.
Asia Pacific will be the growing region for the HPV testing and Pap test market during the forecast period. The increasing rate of cervical cancer and HPV in major countries is responsible for the rapid PAP market growth.
China and India have the largest incidence globally, with over 100,000 cases of cervical cancer diagnosed every year in China. Furthermore, advancement in technology and increased investment by the government to develop healthcare infrastructure in the region are expected to drive the market growth of pap testing over the study period.
Competitive Insight
The companies are investing heavily in R&D to develop new pap examinations such as blood-based and AI-based HPV testing. Major players are involved in strategic partnerships and acquisitions and raise funds to expand their foothold in the market.
Some of the major players operating in the HPV testing and Pap test market include Abbott Laboratories, Arbor Vita Corporation, Becton, BioMérieux SA., Danaher, Dickinson and Company, Femasys, Inc., Fujirebio Diagnostics, Inc., Hologic, Inc., NURX, Inc., Onco Health Corporation, Qiagen, Quest Diagnostics, Roche Ltd, Seegene, Inc., Takara Bio Inc., Thermo Fisher Scientific, Inc., TruScreen
HPV Testing and Pap Test Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2020 |
USD 1.75 billion |
|
Revenue forecast in 2028 |
USD 6.39 billion |
|
CAGR |
15.9% from 2021 - 2028 |
|
Base year |
2020 |
|
Historical data |
2016 - 2019 |
|
Forecast period |
2021 - 2028 |
|
Quantitative units |
Revenue in USD billion and CAGR from 2021 to 2028 |
|
Segments covered |
By Test, By Application, By Product, By Technology, By Region |
|
Regional scope |
North America Europe Asia Pacific Latin America, Middle East & Africa |
|
Key Companies |
Abbott Laboratories, Arbor Vita Corporation, Becton, BioMérieux SA., Danaher, Dickinson and Company, Femasys, Inc., Fujirebio Diagnostics, Inc., Hologic, Inc., NURX, Inc., Onco Health Corporation, Qiagen, Quest Diagnostics, Roche Ltd, Seegene, Inc., Takara Bio Inc., Thermo Fisher Scientific, Inc., TruScreen. |
Want to check out the Hpv Testing And Pap Test Market industry report before buying it? Then, our sample report has got you covered. It includes key market data points, ranging from trend analyses to industry estimates and forecasts. See for yourself by downloading the sample report.
