
eClinical Solutions Market Size, Share, Trends, Industry Analysis Report: By Product, Delivery Mode (Cloud & Web-Based and On-Premise), Clinical Trial, End Use, and Region (North America, Europe, Asia Pacific, Latin America, and Middle East & Africa) – Market Forecast, 2025–2034
- Published Date:Apr-2025
- Pages: 115
- Format: PDF
- Report ID: PM1093
- Base Year: 2024
- Historical Data: 2020-2023
eClinical Solutions Market Overview
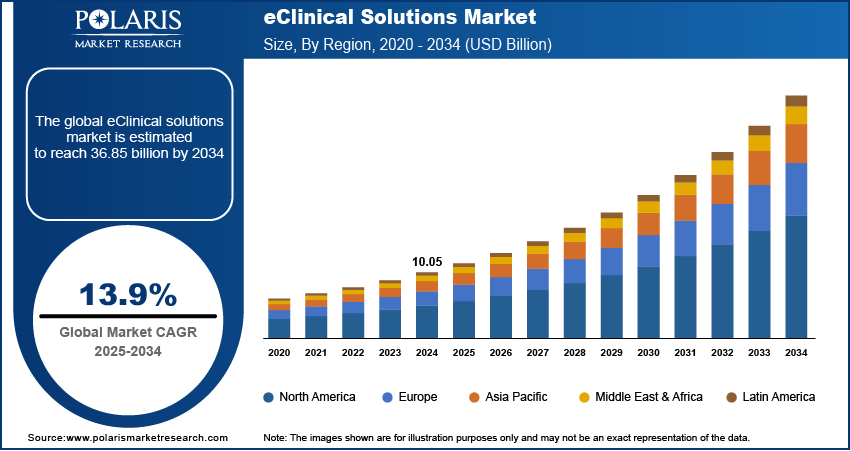
The global eClinical solutions market size was valued at USD 10.05 billion in 2024. The market is projected to grow from USD 11.42 billion in 2025 to USD 36.85 billion by 2034, exhibiting a CAGR of 13.9% during 2025–2034.
The eClinical solutions market comprises software and services that support the electronic management of clinical trials, including data capture, trial management, and analytics. The eClinical solutions market growth is driven by the increasing adoption of digital technologies in clinical research, the growing complexity of clinical trials, and the need for efficient data management and regulatory compliance. Key trends include the integration of artificial intelligence (AI) and machine learning (ML) for data analysis, the rising use of decentralized and virtual clinical trials, and the increasing adoption of cloud-based platforms for real-time data access and collaboration. The market is expected to expand as pharmaceutical companies and contract research organizations seek to improve trial efficiency and reduce costs.
To Understand More About this Research: Request a Free Sample Report
eClinical Solutions Market Dynamics
Adoption of Digital Technologies in Clinical Research
The adoption of electronic data capture (EDC) systems, clinical trial management systems (CTMS), and electronic clinical outcome assessment (eCOA) tools enhances data accuracy and streamlines trial workflows. For instance, the use of cloud-based platforms allows researchers and clinicians to access data and applications from anywhere, promoting collaboration and efficiency. These platforms also offer advanced security features and automatic updates, ensuring compliance with the latest regulations and protection against data breaches. Thus, the increasing integration of digital technologies into clinical research processes is driving the eClinical solutions market growth.
Growing Complexity of Clinical Trials
The increasing complexity of clinical trials necessitates advanced data management solutions, thereby driving the demand for eClinical tools. Modern clinical trials often involve large volumes of data from diverse sources, including genomics, imaging, and wearable devices. Managing this data efficiently requires sophisticated systems capable of integration and analysis. The complexity is further compounded by the need to adhere to stringent regulatory requirements, making comprehensive eClinical solutions essential for successful trial execution.
Rising Emphasis on Regulatory Compliance and Data Security
The stringent regulatory environment governing clinical trials underscores the importance of compliance and data security, propelling the adoption of eClinical solutions. Regulatory frameworks, such as the FDA's 21 CFR Part 11, mandate strict controls over electronic records and signatures, necessitating robust data management systems. eClinical solutions offer advanced security features, audit trails, and automated compliance checks, ensuring that clinical trial processes meet regulatory standards and maintain data integrity. As a result, the rising emphasis on regulatory compliance and data security is propelling the eClinical solutions market revenue.

eClinical Solutions Market Segment Insights
eClinical Solutions Market Assessment by Product Insights
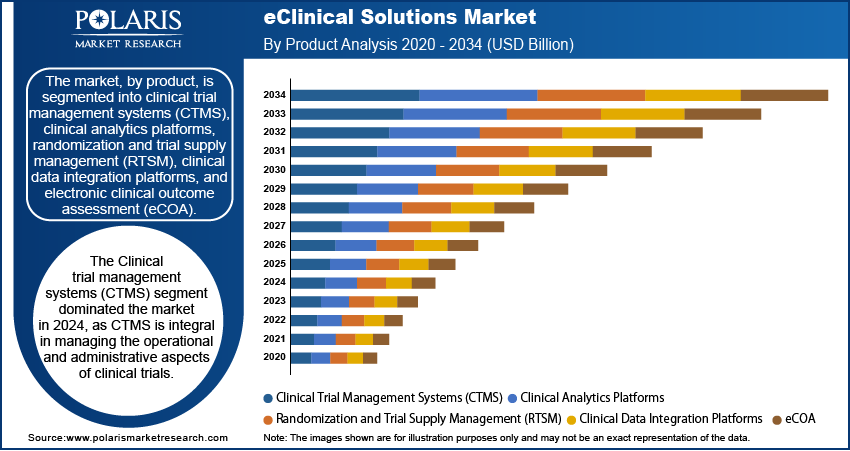
The eClinical solutions market, by product, is segmented into clinical trial management systems (CTMS), clinical analytics platforms, randomization and trial supply management (RTSM), clinical data integration platforms, and electronic clinical outcome assessment (eCOA). Clinical trial management systems (CTMS) have emerged as the leading segment, accounting for the largest revenue share of 20.62% in 2024. CTMS are integral in managing the operational and administrative aspects of clinical trials, including project management and financial oversight. Their widespread adoption by research sites, sponsors, and contract research organizations (CROs) underscores their critical role in streamlining clinical trial processes.
The electronic clinical outcome assessment (eCOA) segment is anticipated to experience the fastest growth from 2025 to 2034, driven by the increasing importance of high-quality clinical data. eCOA platforms enhance data collection by capturing outcomes reported by clinicians, patients, and observers, thereby improving data accuracy and analysis. The ability of eCOA solutions to streamline data collection procedures and maintain the integrity of captured information makes them increasingly valuable in clinical research.
eClinical Solutions Market Evaluation by Delivery Mode Insights
The eClinical solutions market, by delivery mode, is segmented into cloud & web-based and on-premise. The cloud & web-based segment led the market with 91.3% of the revenue share in 2024. This prominence is attributed to benefits such as easy accessibility, usability, and lower investment requirements. Web and cloud-based solutions offer enhanced interoperability and customization, allowing providers to tailor information presentation for different user groups. These advantages have led to widespread adoption across various stakeholders in clinical research.
The on-premise delivery mode, while representing a smaller market share, remains a preferred choice for organizations prioritizing data security and control. Installing services and solutions on internal servers provides complete access to information and full control within the organization's premises. Despite requiring installation within the organization's infrastructure, on-premise solutions can be accessed remotely, offering benefits such as reduced costs due to power consumption and system maintenance. This delivery mode is favored by entities that manage sensitive data and require stringent security measures.
eClinical Solutions Market Outlook by End Use Insights
The eClinical solutions market, by end use, is segmented into hospitals/healthcare providers, contract research organizations (CROs), academic institutes, pharma & biotech organizations, and others. The contract research organizations (CROs) segment dominated the market with the largest revenue share of 37.1% in 2024. This dominance is attributed to the increasing collaboration between biopharmaceutical companies and CROs, as firms seek to reduce overall expenditures and enhance research efficiency. The outsourcing of clinical trials to CROs offers benefits such as specialized expertise and streamlined processes, further driving the adoption of eClinical solutions within this segment.
The pharma & biotech organizations segment is anticipated to experience significant growth during the forecast period. The segment’s robust growth is driven by substantial investments in drug development and approvals, as well as the increasing number of clinical trials conducted globally. The need for efficient management of clinical trial data and compliance with regulatory standards further propels the demand for eClinical solutions among these organizations.
eClinical Solutions Market Regional Analysis
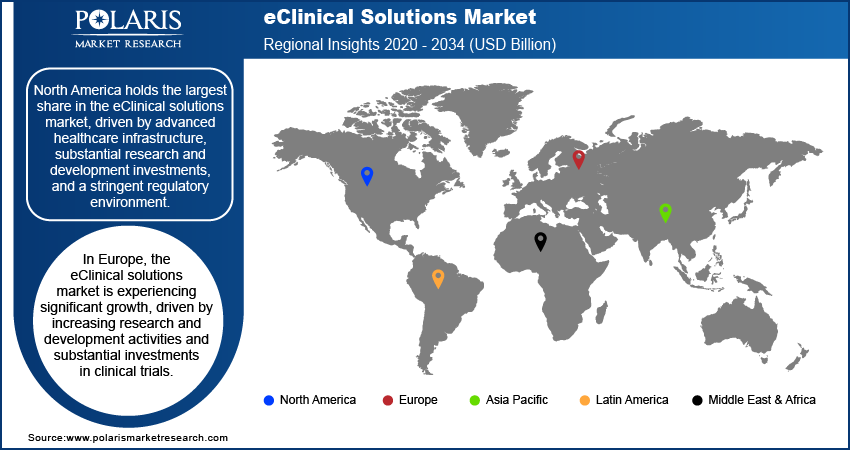
By region, the study provides eClinical Solutions market insights into North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. North America holds the largest share in the eClinical solutions market, driven by advanced healthcare infrastructure, substantial research and development investments, and a stringent regulatory environment. The presence of major pharmaceutical companies conducting extensive clinical trials further fuels the demand for eClinical solutions in the region.
In Europe, the eClinical solutions market is experiencing significant growth, driven by increasing research and development activities and substantial investments in clinical trials. The region's advanced healthcare infrastructure and the establishment of sophisticated laboratories further contribute to this expansion.
The Asia Pacific eClinical solutions market is anticipated to exhibit the fastest growth during the projection period. Factors such as a large patient population, an increase in clinical research activities, and the rapid adoption of advanced technologies in countries like Japan, South Korea, and Australia are key drivers of the region’s robust growth.
eClinical Solutions Market – Key Players and Competitive Insights
The eClinical solutions market features several prominent companies actively contributing to advancements in clinical trial technologies. Medidata Solutions, a subsidiary of Dassault Systèmes, offers cloud-based solutions for clinical trials. Veeva Systems provides cloud-based software tailored for the life sciences industry. IQVIA Inc. delivers technology solutions and contract research services. ICON plc specializes in clinical research and development services. Oracle Corporation offers comprehensive cloud applications and platform services. Signant Health focuses on patient-centric clinical trial solutions. Clario provides clinical trial endpoint technology solutions. eClinical Solutions LLC offers digital clinical software and services. PAREXEL International Corporation provides biopharmaceutical services. CRF Health, now part of Signant Health, specializes in patient-centered eSource technology solutions. ERT Clinical, now part of Clario, offers data collection solutions for clinical trials. Anju Software provides comprehensive software solutions for the life sciences industry.
In the competitive landscape, companies are focusing on strategic acquisitions and partnerships to enhance their service offerings and expand market reach. For instance, Signant Health's acquisition of DSG aims to strengthen its eClinical solutions suite for both traditional and decentralized clinical trials. Similarly, Almac Clinical Technologies' acquisition of Your Research is intended to expand its eClinical solutions for participants, sites, and sponsors. These strategic moves reflect a broader industry trend toward consolidation and integration of advanced technologies to improve clinical trial efficiency and data management.
Additionally, investments from private equity firms are playing a significant role in the growth and development of eClinical solution providers. eClinical Solutions LLC's recent majority investment from GI Partners is expected to further its mission of accelerating clinical trials through digital innovation. Such financial backing enables companies to invest in research and development, expand their technological capabilities, and better serve the evolving needs of the clinical research industry.
Medidata Solutions, a subsidiary of Dassault Systèmes, provides cloud-based software for clinical trials, including data capture, study management, and analytics. Their platform supports various aspects of clinical research, enhancing data quality and operational efficiency.
Veeva Systems offers cloud-based solutions tailored for the life sciences industry, encompassing areas such as clinical data management, regulatory affairs, and quality control. Their applications aim to streamline processes and ensure compliance with industry standards.
List of Key Companies in eClinical Solutions Market
- Anju Software
- ArisGlobal LLC
- Clario
- CRF Health (now part of Signant Health)
- DATATRAK International, Inc.
- eClinical Solutions LLC
- ERT Clinical (now part of Clario)
- ICON plc
- IQVIA Inc.
- Medidata Solutions (a subsidiary of Dassault Systèmes)
- Medrio, Inc.
- Oracle Corporation
- PAREXEL International Corporation
- Signant Health
- Veeva Systems
eClinical Solutions Industry Developments
- In February 2025, Medidata showcased new study experience capabilities for sites and sponsors at the SCOPE 2025 conference. These advancements were intended to reduce study design and execution timelines.
- In January 2025, Medidata (A Dassault Systèmes Company) (France) Medidata has launched Clinical Data Studio, an AI-powered software platform designed to modernize clinical trial data management.
eClinical Solutions Market Segmentation
By Product Outlook
- Clinical Trial Management Systems (CTMS)
- Clinical Analytics Platforms
- Randomization and Trial Supply Management (RTSM)
- Clinical Data Integration Platforms
- Electronic Clinical Outcome Assessment (eCOA)
By Delivery Mode Outlook
- Cloud & Web-Based
- On-Premise
By Clinical Trial Outlook
- Phase I
- Phase II
- Phase III
- Phase IV
By End Use Outlook
- Hospitals/Healthcare Providers
- Contract Research Organizations (CROs)
- Academic Institutes
- Pharma & Biotech Organizations
- Others
By Regional Outlook
- North America
- US
- Canada
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Netherlands
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Malaysia
- South Korea
- Indonesia
- Australia
- Vietnam
- Rest of Asia Pacific
- Middle East & Africa
- Saudi Arabia
- UAE
- Israel
- South Africa
- Rest of Middle East & Africa
- Latin America
- Mexico
- Brazil
- Argentina
- Rest of Latin America
eClinical Solutions Market Report Scope
|
Report Attributes |
Details |
|
Market Size Value in 2024 |
USD 10.05 billion |
|
Market Size Value in 2025 |
USD 11.42 billion |
|
Revenue Forecast by 2034 |
USD 36.85 billion |
|
CAGR |
13.9% from 2025 to 2034 |
|
Base Year |
2024 |
|
Historical Data |
2020–2023 |
|
Forecast Period |
2025–2034 |
|
Quantitative Units |
Revenue in USD billion and CAGR from 2025 to 2034 |
|
Report Coverage |
Revenue Forecast, Market Competitive Landscape, Growth Factors, and Trends |
|
Segments Covered |
|
|
Regional Scope |
|
|
Competitive Landscape |
|
|
Report Format |
|
|
Customization |
Report customization as per your requirements with respect to countries, regions, and segmentation. |
How is the report valuable for an organization?
Workflow/Innovation Strategy: The eClinical solutions market has been segmented into detailed segments of product, delivery mode, clinical trial, and end use. Moreover, the study provides the reader with a detailed understanding of the different segments at both the global and regional levels.
Growth/Marketing Strategy: The growth strategy in the eClinical solutions market focuses on expanding cloud-based platforms to enhance accessibility, scalability, and cost-efficiency. Companies are increasingly investing in partnerships, acquisitions, and technological innovations to integrate decentralized clinical trials and improve data management capabilities. Market players are also emphasizing AI and ML to optimize clinical trial design, recruitment, and data analysis. Additionally, a shift towards patient-centric solutions is driving new product development, catering to the growing demand for more flexible and efficient trial management systems. This trend is being supported by a rise in collaborations between pharmaceutical companies and technology providers.
FAQ's
The eClinical solutions market size was valued at USD 10.05 billion in 2024 and is projected to grow to USD 36.85 billion by 2034.
The market is projected to register a CAGR of 13.9% from 2025 to 2034.
North America had the largest share of the global market in 2024.
The eClinical solutions market features several prominent companies actively contributing to advancements in clinical trial technologies. Medidata Solutions, a subsidiary of Dassault Systèmes, offers cloud-based solutions for clinical trials. Veeva Systems provides cloud-based software tailored for the life sciences industry. IQVIA Inc. delivers technology solutions and contract research services. ICON plc specializes in clinical research and development services. Oracle Corporation offers comprehensive cloud applications and platform services.
The clinical trial management systems (CTMS) segment accounted for the largest market share in 2024.
The cloud & web-based segment dominated the market in 2024.
eClinical solutions refer to software and technology platforms used to streamline and manage various aspects of clinical trials. These solutions encompass tools for data collection, clinical trial management, analytics, regulatory compliance, patient monitoring, and outcome assessments. eClinical solutions aim to improve the efficiency, accuracy, and speed of clinical research by automating processes, enabling real-time data access, and supporting collaboration among different stakeholders, including sponsors, contract research organizations (CROs), and healthcare providers. These solutions are typically cloud-based, offering scalability and flexibility for researchers across the globe.
A few key trends in the market are described below: Adoption of Cloud-Based Solutions: Growing shift towards cloud-based platforms for better scalability, data accessibility, and cost efficiency. Decentralized Clinical Trials: Increase in the adoption of decentralized trials, driven by the need for more flexible and patient-centric approaches. Artificial Intelligence (AI) and Machine Learning (ML) Integration: Use of AI and ML for optimizing trial design, patient recruitment, data analysis, and predictive modeling. Patient-Centric Solutions: Development of solutions that focus on improving patient engagement, real-time monitoring, and data collection.
A new company entering the eClinical solutions market could focus on developing innovative, cloud-based platforms that emphasize scalability, real-time data access, and integration with electronic health records (EHR) for smoother data exchange. Additionally, prioritizing patient-centric solutions, such as mobile apps and remote monitoring tools, would cater to the increasing demand for decentralized clinical trials. Leveraging AI and ML to enhance trial design, patient recruitment, and data analysis could provide a competitive edge. Furthermore, offering strong cybersecurity features to ensure data privacy and compliance with global regulatory standards will help build trust and credibility in the market.
Companies developing, distributing, or purchasing eClinical Solutions and related products, and other consulting firms must buy the report.
