
Cytomegalovirus Treatment Market Share, Size, Trends, Industry Analysis Report, By Drug (Ganciclovir, Valganciclovir, Cidofovir, Foscarnet, Others); By Application; By Distribution Channel; By Region; Segment Forecast, 2024- 2032
- Published Date:Jan-2024
- Pages: 115
- Format: PDF
- Report ID: PM4417
- Base Year: 2023
- Historical Data: 2019 – 2022
Report Outlook
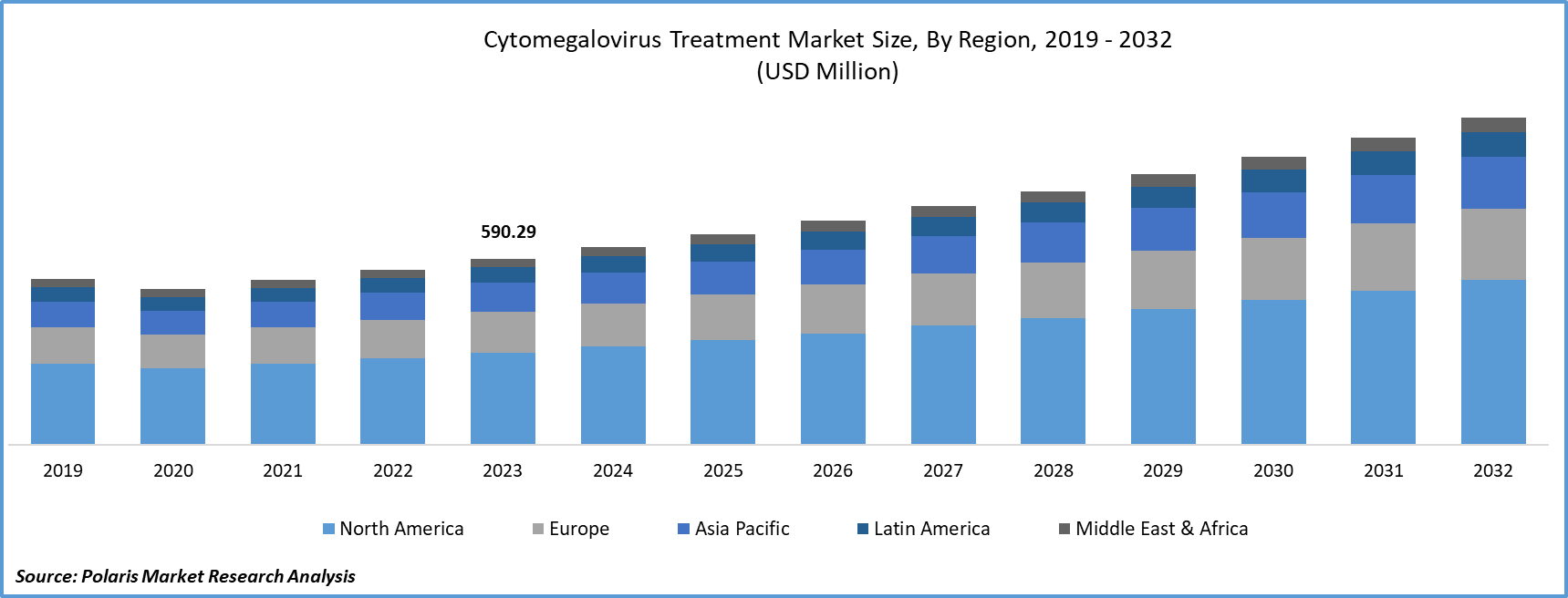
- Cytomegalovirus Treatment Market size was valued at USD 590.29 million in 2023.
- The market is anticipated to grow from USD 627.36 million in 2024 to USD 1,035.16 million by 2032, exhibiting the CAGR of 6.5% during the forecast period.
Market Introduction
Global initiatives to combat infectious diseases are fueling the growth of the cytomegalovirus (CMV) treatment market. With an increased focus on public health, there is a rising demand for effective treatments against CMV infections. Antiviral drugs like ganciclovir and valganciclovir are gaining prominence in the market as CMV remains a significant global health concern. Ongoing research, international collaborations, and concerted efforts to address infectious diseases worldwide are fostering innovation in CMV treatment options. The market's expansion underscores a commitment to enhancing healthcare outcomes globally, particularly in the context of infectious diseases, solidifying its role in the broader landscape of global health.
In addition, companies operating in the market are being granted approvals for new solutions to cater to the growing market demand.
- For instance, in December 2023, Takeda revealed that LIVTENCITY (maribavir) received approval from China's National Medical Products Administration (NMPA) for treating adult individuals with cytomegalovirus (CMV) disease post-hematopoietic stem cell transplant or solid organ transplant. This approval applies specifically to cases where the CMV disease has proven resistant to treatment with ganciclovir, valganciclovir, cidofovir, or foscarnet.
Rising global healthcare expenditure is a catalyst driving the cytomegalovirus treatment market share. Increased financial allocations to healthcare, especially for infectious diseases like CMV, are evident as budgets expand. The surge in immunocompromised populations, coupled with a growing emphasis on advanced antiviral therapies such as Ganciclovir, aligns with the heightened healthcare spending. Additionally, enhanced awareness and diagnostics contribute to accurate CMV diagnosis, further fueling the demand for effective treatments. This intersection of elevated healthcare investments, CMV prevalence, and therapeutic advancements defines the market's growth trajectory, fostering innovation and bolstering the overall landscape with improved patient care.
To Understand More About this Research: Request a Free Sample Report
Industry Growth Drivers
- Increasing incidence of cytomegalovirus infections is projected to spur the product demand
The escalating incidence of cytomegalovirus infections is a major driver propelling the cytomegalovirus treatment market size. A notable rise in immunocompromised populations, such as organ transplant recipients and individuals with HIV/AIDS, amplifies the risk, while improved diagnostics enhance detection rates. This surge in cases necessitates effective antiviral treatments like Ganciclovir, fostering market growth. Pharmaceutical and biotechnology firms respond by intensifying research and development efforts, aiming to meet the increasing therapeutic demands. The industry's focus on innovation and the development of novel antiviral agents underscores its commitment to addressing the expanding public health challenge posed by the growing prevalence of CMV infections.
- Rising transplantation activities is expected to drive cytomegalovirus treatment market growth
The cytomegalovirus treatment market industry is flourishing due to a surge in transplantation activities. With an increase in organ and stem cell transplants, the incidence of CMV infections among recipients has risen. This heightened demand for effective CMV treatment options has fueled market expansion. Antiviral drugs like ganciclovir and valganciclovir play a crucial role in managing CMV infections, contributing to market growth. As transplantation procedures become more widespread globally, the demand for advanced and targeted CMV treatments escalates. The market's significance lies in its pivotal role in addressing the growing healthcare challenge of CMV infections in the context of increasing transplantation activities.
Industry Challenges
- High cost of treatment is likely to impede the market growth
Expenses related to antiviral medications, diagnostics, and supportive care create a financial burden, restricting access to effective CMV treatments. This economic challenge particularly affects vulnerable populations and healthcare systems with budget constraints, impacting treatment adherence and patient outcomes. Collaborative efforts are essential among pharmaceutical companies, healthcare providers, and policymakers to develop cost-effective solutions. Overcoming the hurdle of high treatment costs is imperative for widening access to CMV treatments and improving overall intervention efficacy against this viral infection.
Report Segmentation
The cytomegalovirus treatment market analysis is primarily segmented based on drug, application, distribution channel, and region.
|
By Drug |
By Application |
By Distribution Channel |
By Region |
|
|
|
|
To Understand the Scope of this Report: Speak to Analyst
By Drug Analysis
Ganciclovir segment accounted for significant market share in 2023
The ganciclovir segment accounted for a significant market share in 2023. Multifaceted factors propel Ganciclovir's widespread adoption in cytomegalovirus treatment. Renowned for potent antiviral efficacy targeting CMV DNA polymerase, it proves pivotal across diverse clinical scenarios, notably in immunocompromised patients. Its prophylactic significance, formulation flexibility with intravenous and oral options, and integration into comprehensive treatment strategies highlight its versatility. Inclusion in established guidelines, prevention of CMV-associated end-organ damage, rapid onset benefits, and a robust risk-benefit profile further solidify its role. Extensive research validation, especially in high-risk contexts like organ transplantation, ensures confidence in its safety and efficacy, emphasizing patient adherence through available oral formulations.
By Application Analysis
Stem cell transplantation segment accounted for significant market share in 2023
The stem cell transplantation segment accounted for a significant market share in 2023. Stem cell transplant recipients face an elevated risk of CMV reactivation due to immunosuppression, necessitating the use of antiviral medications like ganciclovir and valganciclovir for prevention and treatment. Regular blood monitoring is crucial to detect CMV reactivation, enabling prompt intervention to prevent complications ranging from mild symptoms to severe organ damage. Ongoing research in stem cell transplantation aims to optimize CMV treatment strategies, focusing on efficacy, minimizing side effects, and improving overall outcomes for transplant recipients.
By Distribution Channel Analysis
Hospital pharmacy segment held significant market revenue share in 2023
The hospital pharmacy segment held a significant revenue share in 2023. Hospitals employ advanced diagnostic tests for precise CMV detection, with ongoing monitoring through blood tests and imaging. A diverse array of antiviral medications, including ganciclovir and valganciclovir, is administered intravenously, ensuring controlled delivery and close medical supervision. Specialized care units cater to severe cases, emphasizing tailored approaches for transplant recipients. Multidisciplinary collaboration, encompassing infectious disease specialists and transplant teams, enhances care coordination. Hospitals prioritize patient education on medication adherence and potential side effects, while continuous evaluation guides adaptive treatment plans. Isolation protocols, if necessary, ensure a controlled environment, emphasizing a patient-centric and comprehensive hospital-based CMV treatment paradigm.
Regional Insights
North America region dominated the global market in 2023
In 2023, the North American region dominated the global market. The North American market features a diverse ecosystem of pharmaceutical and biotechnology players, research institutions, and dynamic market dynamics. Epidemiological considerations focus on high-risk populations, while treatment strategies encompass a range of antiviral modalities, including intravenous and oral approaches. Ongoing research and innovation drive the development of novel therapies, with a robust regulatory framework overseen by entities such as the FDA and Health Canada. Key market players engage in strategic collaborations to solidify their positions, and North America's prominence in clinical trials fosters continual advancements. Initiatives addressing patient access, affordability, and emerging therapeutic paradigms underscore the market's dynamic evolution, shaping trends toward personalized medicine and innovative combination therapies.
Over the projected period, the Asia-Pacific region is likely to experience significant growth. The cytomegalovirus treatment industry in Asia-Pacific is witnessing unique trends and challenges specific to the region. With a diverse population and varied healthcare infrastructures, Asia grapples with distinct epidemiological patterns of CMV infections. The demand for effective treatment options is driven by factors such as a high prevalence of CMV in certain Asian populations and the increasing awareness of the virus. Regional regulatory landscapes, healthcare policies, and economic conditions influence the industry.
Key Market Players & Competitive Insights
The market is marked by a diverse array of participants, and the anticipated entry of numerous new players is set to intensify the competitive landscape. Well-established leaders in the industry continuously upgrade their technologies, aiming to sustain a competitive edge through a focus on efficiency, integrity, and safety. These entities prioritize strategic initiatives such as establishing partnerships, enhancing product offerings, and engaging in collaborative ventures to outperform their peers. The overarching objective is to secure a significant cytomegalovirus treatment market share.
Some of the major players operating in the global market include:
- Bayer AG
- Bristol Myers Squibb
- Chimerix
- Clinigen Group
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd
- Fresenius Kabi
- Genentech, Inc.
- Gilead Sciences, Inc.
- GlaxoSmithKline plc
- Merck & Co., Inc.
- Pfizer Inc.
- Takeda Pharmaceuticals
- Teva Pharmaceutical Industries Ltd.
- Thermo Fisher Scientific
Recent Developments
- In March 2023, Mitsubishi Tanabe Pharma Corporation declared that it obtained approval for an extra indication for the anti-cytomegalovirus agent ValixaDry Syrup 5000mg. This approval pertains to the treatment of symptomatic congenital cytomegalovirus infection.
- In February 2023, Merck disclosed that the U.S. Food and Drug Administration agreed to assess two supplemental new drug applications (sNDA) for PREVYMIS (letermovir). The FDA has accorded priority review for the sNDA concerning PREVYMIS's prophylactic use in preventing cytomegalovirus (CMV) disease in high-risk adult kidney transplant recipients.
- In May 2022, Hologic, Inc. reported that the U.S. Food and Drug Administration provided approval for its Aptima CMV Quant assay. This assay is designed to measure the viral load of cytomegalovirus (CMV) in individuals who underwent solid organ or stem cell transplants.
Report Coverage
The cytomegalovirus treatment market report emphasizes on key regions across the globe to provide better understanding of the product to the users. Also, the report provides market insights into recent developments, trends and analyzes the technologies that are gaining traction around the globe. Furthermore, the report covers in-depth qualitative analysis pertaining to various paradigm shifts associated with the transformation of these solutions.
The report provides detailed analysis of the market while focusing on various key aspects such as competitive analysis, drugs, applications, distribution channels, and their futuristic growth opportunities.
Cytomegalovirus Treatment Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2024 |
USD 627.36 million |
|
Revenue forecast in 2032 |
USD 1,035.16 million |
|
CAGR |
6.5% from 2024 – 2032 |
|
Base year |
2023 |
|
Historical data |
2019 – 2022 |
|
Forecast period |
2024 – 2032 |
|
Quantitative units |
Revenue in USD million and CAGR from 2024 to 2032 |
|
Segments covered |
|
|
Regional scope |
|
|
Competitive Landscape |
|
|
Report Format |
|
|
Customization |
Report customization as per your requirements with respect to countries, region and segmentation. |
Explore the 2024 market share, size, and revenue growth rate statistics in the field of Cytomegalovirus Treatment Market, meticulously compiled by Polaris Market Research Industry Reports. This comprehensive analysis encompasses a market forecast outlook extending to 2032, along with an insightful historical overview. Experience the depth of this industry analysis by obtaining a complimentary PDF download of the sample report.
Browse Our Top Selling Reports:
Rye Market Size, Share 2024 Report
Packaged Salad Market Size, Share 2024 Report
Coworking Spaces Market Size, Share 2024 Report
FAQ's
The Cytomegalovirus Treatment Market report covering key segments are drug, application, distribution channel, and region.
Cytomegalovirus Treatment Market Size Worth $1,035.16 Million By 2032
Cytomegalovirus Treatment Market exhibiting the CAGR of 6.5% during the forecast period.
North America is leading the global market
key driving factors in Cytomegalovirus Treatment Market are increasing incidence of cytomegalovirus infections is projected to spur the product demand
