
Clinical Trial Biorepository & Archiving Solutions Market Size, Share, Trends, Industry Analysis Report: By Product (Preclinical Products and Clinical Products), By Service, By Phase, and By Region (North America, Europe, Asia Pacific, Latin America, and Middle East & Africa) – Market Forecast, 2024–2032
- Published Date:Sep-2024
- Pages: 116
- Format: PDF
- Report ID: PM5040
- Base Year: 2023
- Historical Data: 2019-2022
Clinical Trial Biorepository & Archiving Solutions Market Overview
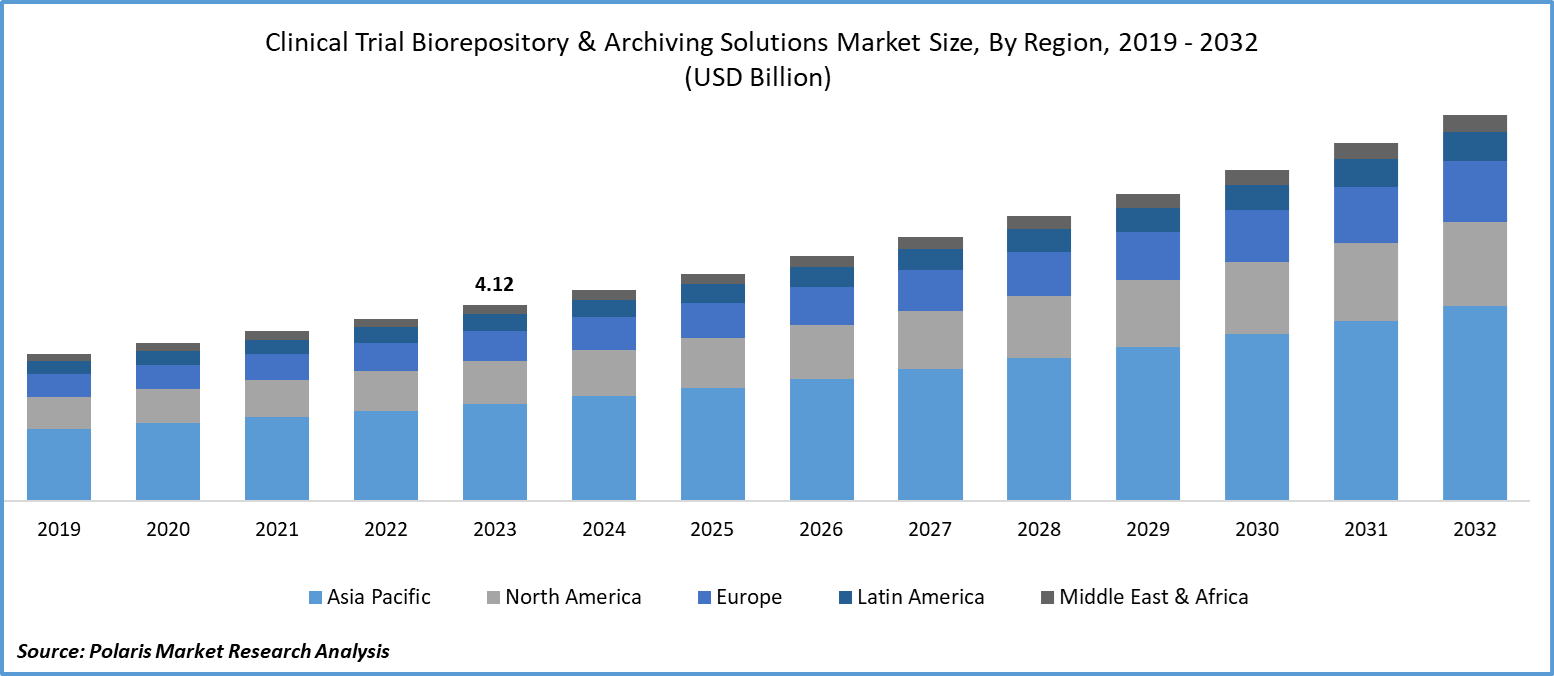
The global clinical trial biorepository & archiving solutions market size was valued at USD 4.12 billion in 2023. The market is projected to grow from USD 4.43 billion in 2024 to USD 8.09 billion by 2032, exhibiting a CAGR of 7.8% during 2024–2032.
The global clinical trial biorepository & archiving solutions market is experiencing robust growth driven by increasing investments in drug development and the rising demand for personalized medicine. Key drivers include the expanding number of clinical trials, which necessitate sophisticated biorepository solutions to manage and store large volumes of biological samples efficiently. Additionally, advancements in biobanking technologies and regulatory requirements for data integrity and sample traceability fuel the market growth. Trends such as the integration of automated systems for sample processing and the growing emphasis on data analytics for research insights are shaping the industry landscape. As biobanks become integral to advancing clinical research and precision medicine, the clinical trial biorepository & archiving solutions market is poised for continued expansion and innovation.
To Understand More About this Research: Request a Free Sample Report
Clinical Trial Biorepository & Archiving Solutions Market Trends
Expansion of Automated Sample Management
The integration of automated systems in biorepositories is a major trend transforming the global clinical trial biorepository & archiving solutions market. Automated sample management systems enhance efficiency by streamlining the processes of sample handling, storage, and retrieval. These systems reduce the risk of human error, ensure consistent and precise sample tracking, and facilitate high-throughput processing. The automation trend is driven by the need to manage large volumes of biological samples efficiently and with high accuracy. As the complexity of clinical trials increases and the number of samples grows, automated solutions are becoming essential for maintaining the integrity and accessibility of biorepository collections.
Growing Focus on Data Analytics and Integration
The emphasis on data analytics is reshaping biorepository and archiving solutions by enabling more sophisticated analysis and integration of clinical trial data. Advanced analytics tools are being employed to derive actionable insights from biological samples, supporting personalized medicine and precision research. This trend includes the use of bioinformatics platforms that integrate data from various sources, such as genomic, proteomic, and clinical data, to enhance research outcomes. The drive toward data-driven decision-making is pushing biorepositories to adopt integrated systems that facilitate real-time data access and analysis, thus enhancing the overall efficiency and value of clinical trials.
Increasing Regulatory and Compliance Requirements
Regulatory and compliance requirements are becoming increasingly stringent, significantly influencing the clinical trial biorepository & archiving solutions market. Global standards and regulations set by regulatory bodies, such as the Food and Drug Administration (FDA) and European Medicines Agency (EMA), mandate strict guidelines for sample handling, storage, and documentation to ensure data integrity and participant safety. This trend has led to the development of more robust and compliant biorepository systems that incorporate features such as secure data storage, comprehensive tracking, and audit trails. As regulatory bodies continue to enforce rigorous standards, biorepositories must continuously adapt their processes and technologies to meet these evolving requirements, driving market growth and innovation.
Clinical Trial Biorepository & Archiving Solutions Market – Segment Insights
Clinical Trial Biorepository & Archiving Solutions Market – Product Insights
The global clinical trial biorepository & archiving solutions market, by product, is bifurcated into preclinical products and clinical products. Preclinical products, which comprise sample storage and management solutions for early-stage research, typically involve lower volumes and less complex requirements than clinical products. Despite this, the preclinical products segment holds a significant share of the market due to the foundational role they play in drug discovery and development. However, the clinical products segment dominates the market, driven by the expansive growth in clinical trials and the increasing need for robust biorepository solutions that support large-scale sample handling, long-term storage, and compliance with stringent regulatory standards.
The clinical products segment is expected to register a higher growth rate due to the surging clinical trials across the world and the rising emphasis on personalized medicine. The expansion of clinical trials across diverse therapeutic areas necessitates advanced biorepository solutions that can handle complex sample types and large datasets. This growth is fueled by the integration of automated systems and enhanced data analytics capabilities, which address the evolving demands of clinical research. As clinical trials become more extensive and intricate, the market for Clinical Products is set to experience significant growth, outpacing the Preclinical Products segment in both market share and growth rate.
Clinical Trial Biorepository & Archiving Solutions Market – Service Insights
The global clinical trial biorepository & archiving solutions market, based on service, is bifurcated into biorepository services and archiving solution services. The biorepository services segment, which encompasses sample collection, processing, and storage, holds a dominant position in the market due to the increasing demand for comprehensive sample management throughout the clinical trial lifecycle. These services are critical for maintaining the quality and integrity of biological samples, making them essential for researchers and pharmaceutical companies. However, archiving solution services, which focus on long-term storage and preservation of data and samples, represent a vital but smaller segment.
The highest growing segment is biorepository services, driven by the expansion of clinical trials and the need for sophisticated sample management solutions. As clinical research becomes complex and personalized medicine gains prominence, the demand for advanced biorepository services that offer enhanced automation, data integration, and compliance is surging. This growth is supported by technological advancements and the need for more efficient sample handling and tracking systems. While archiving solution services remain crucial for ensuring long-term data and sample preservation, the rapid evolution and increasing scale of clinical trials are propelling biorepository services to be the fastest-growing segment in the market.
Clinical Trial Biorepository & Archiving Solutions Market – Phase Insights
In the global clinical trial biorepository & archiving solutions market, segmentation by phase highlights the distinct needs and dynamics of different stages of clinical trials. The preclinical phase, while critical for early-stage research and foundational studies, represents a smaller segment than the later phases of clinical trials. Among the various phases, the phase III segment dominates the market due to the large-scale nature of these trials, which require extensive sample management and archiving solutions to support large patient cohorts and comprehensive data collection. The volume and complexity of data and samples in phase III trials drive the demand for advanced biorepository solutions to ensure compliance and integrity.
The highest growing segment is phase I, driven by increasing investments in early-stage research and the need for efficient sample handling and data management from the onset of clinical trials. The expansion of personalized medicine and innovative therapeutic approaches fuel the growth in phase I trials, as researchers seek to optimize early data collection and analysis. This surge is prompting a greater demand for biorepository solutions that can cater to the unique requirements of early-phase trials, including precise sample tracking and high-throughput processing capabilities. While phase III remains the largest segment, phase I is experiencing the highest growth rate in the market.
Clinical Trial Biorepository & Archiving Solutions Market, Segmental Coverage, 2019 - 2032 (USD billion)
Source: Secondary Research, Primary Research, PMR Database and Analyst Review
Clinical Trial Biorepository & Archiving Solutions Market Regional Insights
By region, the study provides the market insights into North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. In the global clinical trial biorepository & archiving solutions market, North America is the dominating region due to its advanced healthcare infrastructure, significant investments in clinical research, and high concentration of pharmaceutical and biotechnology companies. The region's leadership is driven by its robust clinical trial ecosystem, extensive network of biorepositories, and stringent regulatory frameworks that ensure high standards of sample management and data integrity. Additionally, North America's strong emphasis on innovation and technology adoption, coupled with substantial funding for research and development, supports its dominance in the market. While other regions such as Europe and Asia Pacific are growing rapidly and contributing significantly to market expansion, North America's established infrastructure and leadership in clinical research continue to make it the most influential region in the global clinical trial biorepository & archiving solutions landscape.
Clinical Trial Biorepository & Archiving Solutions Market Regional Coverage, 2019 - 2032 (USD billion)
Source: Secondary Research, Primary Research, PMR Database and Analyst Review
In Europe, the global clinical trial biorepository & archiving solutions market benefits from a well-established clinical research infrastructure and a strong emphasis on regulatory compliance. The region's diverse healthcare systems and robust support for research and development propel the demand for sophisticated biorepository and archiving solutions. The market growth in the region is supported by significant investments in medical research, collaborations between academic institutions and industry, and stringent data protection regulations such as GDPR, which influence biorepository practices. The European market also benefits from increasing clinical trial activities and advancements in personalized medicine, contributing to its steady expansion.
The global clinical trial biorepository & archiving solutions market in Asia Pacific is experiencing rapid growth due to its expanding clinical trial landscape and increasing investments in healthcare infrastructure. Countries such as China and India are becoming major hubs for clinical research, driven by large patient populations, growing healthcare investments, and a rising presence of pharmaceutical and biotechnology companies. The region's market growth is further accelerated by favorable regulatory environments, cost-effective research opportunities, and advancements in biobanking technologies. As clinical trials and research activities expand across Asia Pacific, the demand for advanced biorepository solutions continues to surge, positioning the region as a key player in the global market.
Clinical Trial Biorepository & Archiving Solutions Market – Key Market Players & Competitive Insights
Key players in the global clinical trial biorepository & archiving solutions market are Thermo Fisher Scientific, LabCorp, BioStorage Technologies, Brooks Life Sciences, and QIAGEN. Other notable companies are VWR International (part of Avantor), Sartorius AG, Hamilton Company, Precision Biobank, and CryoPort. Companies such as BioBanking Solutions, biorepository and archiving divisions of major pharmaceutical firms such as Pfizer and Novartis, and specialized providers, including Celerion and STC Biologics, are influential in the market.
Competitive analysis reveals that major players in the market are focusing on technological advancements and comprehensive service offerings to maintain a competitive edge. Companies such as Thermo Fisher Scientific and LabCorp leverage their extensive product portfolios and global presence to cater to diverse biorepository needs, including automation and advanced data management. Meanwhile, specialized firms such as Brooks Life Sciences and CryoPort emphasize their expertise in cryogenic storage and logistics, addressing requirements for sample preservation and transportation.
The clinical trial biorepository & archiving solutions market is characterized by a blend of established players and innovative new entrants. Competitive strategies include mergers and acquisitions, strategic partnerships, and investments in R&D to enhance biorepository technologies and expand service capabilities. The growing emphasis on personalized medicine and the increasing volume of clinical trials are driving demand for advanced biorepository solutions, prompting key players to continually innovate and adapt their offerings. As the market evolves, companies that effectively integrate automation, data analytics, and compliance features will be well-positioned to lead in this dynamic and expanding field.
Thermo Fisher Scientific is a major player in the global clinical trial biorepository & archiving solutions market, known for its comprehensive range of biorepository products and services. The company offers advanced solutions for sample storage, management, and processing, supported by its global network and strong focus on innovation. Thermo Fisher Scientific's expertise in automation and data integration enhances its position as a key provider in the market.
Brooks Life Sciences is another major player, specializing in sample storage and management solutions with a focus on biobanking and cryogenic storage technologies. Brooks Life Sciences is recognized for its high-quality, secure, and scalable solutions tailored for clinical trials and research needs. The company's services are designed to support complex and large-scale biorepository operations.
Key Companies in Clinical Trial Biorepository & Archiving Solutions Market
- Thermo Fisher Scientific
- LabCorp
- BioStorage Technologies
- Brooks Life Sciences
- QIAGEN
- VWR International (part of Avantor)
- Sartorius AG
- Hamilton Company
- Precision Biobank
- CryoPort
- BioBanking Solutions
- Celerion
- STC Biologics
- Pfizer (biorepository and archiving division)
- Novartis (biorepository and archiving division)
Clinical Trial Biorepository & Archiving Solutions Industry Developments
- In July 2024, Thermo Fisher Scientific announced the acquisition of a biorepository solutions company, expanding its capabilities in sample management and data analytics. This strategic move aims to enhance its market position and offer more integrated solutions for clinical trials and research.
Clinical Trial Biorepository & Archiving Solutions Market Segmentation
By Product Outlook (Revenue – USD Billion, 2019–2032)
-
Preclinical Products
-
Clinical Products
By Service Outlook (Revenue – USD Billion, 2019–2032)
-
Biorepository Services
-
Archiving Solution Services
By Phase Outlook (Revenue – USD Billion, 2019–2032)
-
Preclinical
-
Phase I
-
Phase II
-
Phase III
-
Phase IV
By Regional Outlook (Revenue – USD Billion, 2019–2032)
- North America
- US
- Canada
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Netherlands
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Malaysia
- South Korea
- Indonesia
- Australia
- Rest of Asia Pacific
- Middle East & Africa
- Saudi Arabia
- UAE
- Israel
- South Africa
- Rest of Middle East & Africa
- Latin America
- Mexico
- Brazil
- Argentina
- Rest of Latin America
Clinical Trial Biorepository & Archiving Solutions Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2023 |
USD 4.12 billion |
|
Market size value in 2024 |
USD 4.43 billion |
|
Revenue forecast in 2032 |
USD 8.09 billion |
|
CAGR |
7.8% from 2024 to 2032 |
|
Base year |
2023 |
|
Historical data |
2019–2022 |
|
Forecast period |
2024–2032 |
|
Quantitative units |
Revenue in USD billion and CAGR from 2024 to 2032 |
|
Report coverage |
Revenue Forecast, Market Competitive Landscape, Growth Factors, and Trends |
|
Segments covered |
|
|
Regional scope |
|
|
Competitive landscape |
|
|
Report format |
|
|
Customization |
Report customization as per your requirements with respect to countries, region, and segmentation. |
How the report is valuable for an organization?
Workflow/Innovation Strategy: The clinical trial biorepository & archiving solutions market has been segmented on the basis of phase, service type, and products. Moreover, the study provides the reader with a detailed understanding of the different segments at both global and regional level.
Growth/Marketing Strategy: The growth and marketing strategy in the global clinical trial biorepository & archiving solutions market centers on technological innovation, strategic partnerships, and expanding global footprints. Companies are focusing on integrating automation and advanced data analytics into their biorepository solutions to meet the growing demands of personalized medicine and large-scale clinical trials. Additionally, mergers and acquisitions are common, enabling firms to broaden their service offerings and enhance market reach. Targeted marketing efforts emphasize compliance with stringent regulatory standards, the reliability of sample management, and the ability to support complex, multi-center clinical trials. These strategies are designed to capture a larger share of the expanding market while addressing the evolving needs of pharmaceutical and biotech clients.
FAQ's
The global clinical trial biorepository & archiving solutions market size was valued at USD 4.12 billion in 2023 and is projected to grow to USD 8.09 billion by 2032.
The global market is projected to register a CAGR of 7.8% during 2023–2032.
North America held the largest share of the global market in 2023.
Thermo Fisher Scientific, LabCorp, BioStorage Technologies, Brooks Life Sciences, and QIAGEN are a few of the key players in the market. Other notable companies are VWR International (part of Avantor), Sartorius AG, Hamilton Company, Precision Biobank, and CryoPort.
The phase III segment dominated the market in 2023.
The biorepository services segment accounted for a largest market share in 2023.
Clinical trial biorepository & archiving solutions refer to specialized services and systems designed to manage, store, and preserve biological samples and associated data collected during clinical trials. These solutions are critical for ensuring the integrity, traceability, and security of samples such as blood, tissue, DNA, and other biological materials, which are essential for research and drug development.
A few key trends in the clinical trial biorepository & archiving solutions market are described below. Automation in Sample Management: Increased adoption of automated systems for efficient and accurate sample handling, storage, and retrieval. Integration of Data Analytics: Growing emphasis on using advanced data analytics to extract actionable insights from biological samples, enhancing research outcomes. Stringent Regulatory Compliance: Increasing regulatory requirements for sample traceability, data integrity, and long-term preservation, driving the need for compliant biorepository solutions.
A new company entering the global clinical trial biorepository & archiving solutions market must focus on areas such as advanced automation and AI-driven data analytics to streamline sample management and enhance research insights. Offering scalable, compliant, and customizable biorepository solutions tailored to emerging needs in personalized medicine and complex clinical trials could differentiate the company.
Companies providing clinical trial biorepository & archiving solutions and related services and other consulting firms must buy the report.
