
Aptamers Market Share, Size, Trends, Industry Analysis Report, By Type (Nucleic Acid, Peptide); By Application (Diagnostics, Therapeutics, Research & Developments, Others); By Region, And Segment Forecasts, 2024 - 2032
- Published Date:Jan-2024
- Pages: 114
- Format: PDF
- Report ID: PM3553
- Base Year: 2023
- Historical Data: 2019-2022
Report Outlook
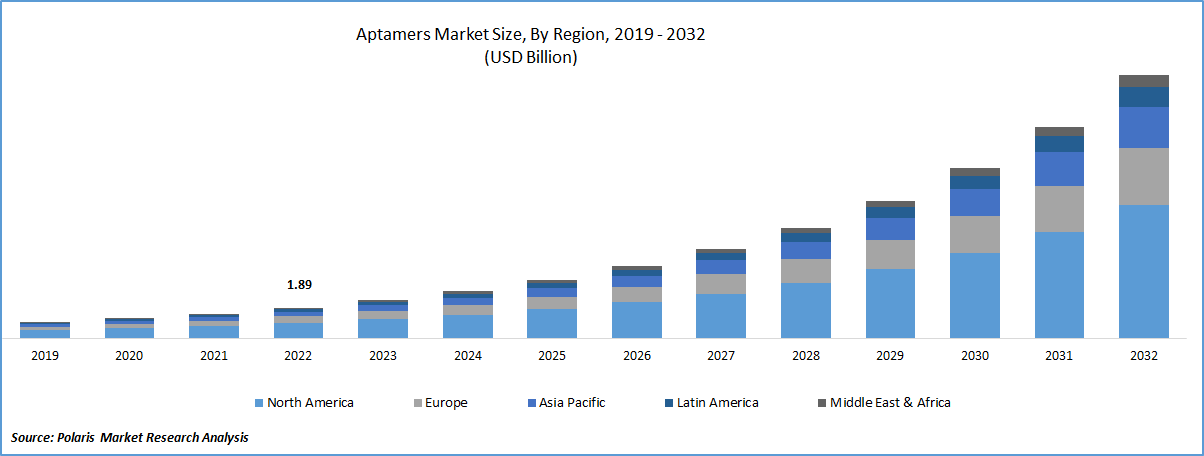
The global aptamers market was valued at USD 2.34 billion in 2023 and is expected to grow at a CAGR of 24.10% during the forecast period. Aptamers have been receiving increased attention as potential therapeutics for various diseases, including COVID-19. Aptamers are short, single-stranded DNA or RNA molecules that can selectively bind to target molecules, such as proteins or cells, with high affinity and specificity. They have several advantages over traditional therapeutics, including antibodies, such as smaller size, higher stability, and lower immunogenicity. In the context of COVID-19, aptamers can potentially be used to target specific viral proteins or host cell receptors involved in the virus's entry or replication. For example, several aptamers have been developed that target the spike protein of SARS-CoV-2, the virus that causes COVID-19, and can inhibit viral entry into host cells.
To Understand More About this Research: Request a Free Sample Report
COVID-19 pandemic has spurred the development of new aptamer-based treatments for the virus, and governments around the world have provided funding for research & development. The Department of Community & Economic Development's award of USD 320,000 to the Aptagen. The hope is that aptamer-based therapies could offer a more effective and targeted treatment option for COVID-19 compared to existing treatments.
Aptamers have been used in the development of diagnostic kits and assays for the detection of SARS-CoV-2 and COVID-19 biomarkers. The SELEX (Systematic Evolution of Ligands by Exponential enrichment) technology is widely used to generate high-affinity aptamers against specific targets, including viral proteins or nucleic acids. Aptamer-based diagnostic kits have several advantages over traditional diagnostic methods, including higher sensitivity, specificity, and speed of detection. They can also be produced at a lower cost and are amenable to large-scale production, making them particularly useful for screening large populations.
The approval of Aptamex by the Ministry of Health of the Republic of Indonesia is a significant milestone in the development of aptamer-based diagnostic kits for COVID-19. Aptamex is a second-generation diagnostic kit that uses DNA aptamers to detect SARS-CoV-2 viral proteins in patient samples. It is cost-effective, chemically synthesized, and can provide results within 30 minutes. Aptamer-based diagnostic products have several advantages over traditional diagnostic methods, making them a preferred choice among diagnostic and pathology labs for the diagnosis of diseases at the cellular level.
Aptamers are small, single-stranded DNA or RNA molecules that can specifically bind to target molecules, such as proteins or cells, with high affinity and selectivity. Due to their small size, aptamers can penetrate tissues more easily, allowing them to target disease biomarkers at the cellular level. The high specificity and selectivity of aptamers also make them ideal for the diagnosis of diseases such as cancer, cardiovascular disease (CVD), and age-related macular degeneration (AMD). For example, aptamers can be used to target specific cancer cells or markers, allowing for early detection and diagnosis of the disease.
Industry Dynamics
Growth Drivers
As the prevalence of these diseases increases, there is a growing demand for accurate and efficient diagnostic tools. Aptamer-based diagnostic products have the potential to meet this demand, as they can provide rapid and sensitive detection of disease biomarkers with high specificity and selectivity. As per the estimate of the UK base Cancer Research firm, around 27.5 Mn individuals across the globe, will suffer from cancer, by 2040.
Macugen, developed by Eyetech Pharmaceuticals is currently the only FDA-approved therapeutic aptamer available in the market. It is used for the treatment of neovascular (wet) age-related macular degeneration (AMD), a leading cause of blindness in older adults. Despite being the only FDA-approved aptamer therapeutic, Macugen has been successful in treating patients with neovascular AMD, demonstrating the potential of aptamers as therapeutic agents. This has encouraged researchers to develop novel aptamer-based drugs for the treatment of various diseases.
One such drug under development is Zimura, developed by IVERIC Bio, Inc., for the treatment of geographic atrophy (GA), a form of dry AMD. Zimura is an aptamer that specifically targets complement factor C5, a key component of the immune system that has been implicated in the development of GA

Report Segmentation
The market is primarily segmented based on type, application, and region.
|
By Type |
By Application |
By Region |
|
|
|
To Understand the Scope of this Report: Speak to Analyst
Nucleic acid segment accounted for the largest market share in 2022
The mechanism of action of nucleic acid aptamers has shown great potential for the treatment of various disorders, including age-related macular degeneration (AMD). There are currently several companies investigating the use of aptamers for the treatment of AMD and other diseases. In June 2021, the U.S. Food and Drug Administration (FDA) granted written agreement under Special Protocol Assessment (SPA) to IVERIC BIO (formerly known as Ophthotech Corporation) for the design of GATHER2 phase 3 clinical trial of Zimura for the treatment of geographic atrophy (GA) secondary to AMD. This is a significant development for the aptamer market, as it demonstrates the potential of aptamers as therapeutic agents for the treatment of AMD.
Peptide aptamer segment projected to witness lucrative growth rate. This is due to the large application base for diagnostic and therapeutic purposes, including cardiovascular disease. In August 2021, researchers at the Engineering Center for Microtechnology and Diagnostics developed new biosensor for the multi-parametric express testing in the pre-clinical diagnostics of the cardiovascular disease. The biosensor was developed using peptide aptamers, which have high specificity and sensitivity for their targets. This is an example of the growing use of peptide aptamers in diagnostic applications, which is expected to drive market growth for this segment.
North America region dominated the global market in 2022
The North America region dominated the global market with considerable market share in 2022. This is primarily due to the rising prevalence of chronic diseases, strong healthcare infrastructure, and growing interest of research laboratories in the aptamer field. The region has a well-established healthcare system, with many diagnostic and research facilities that are actively engaged in developing aptamer-based products for various applications.
In addition, the presence of major market players in the region, such as NOXXON Pharma, has also contributed to the growth of the aptamers market in North America. In April 2022, NOXXON Pharma released its top line results of its “NOX-A12 trial” in the brain cancer represented at the American Society of Clinical Oncology (ASCO) meeting. This highlights the increasing focus on the development of aptamer-based therapeutics for the treatment of cancer, which is one of the major applications of aptamers.
The Asia Pacific region is expected to be the fastest growing region with a healthy CAGR over the study period. Increasing investments by market players, growing research activities, and rising awareness about aptamers-based products are also contributing to the growth of the market. Moreover, the region's large population and the high burden of chronic diseases such as cancer and cardiovascular diseases are creating a favorable environment for the growth of the market.
Competitive Insight
Major players in the aptamers market are continuously focusing on product development and commercialization to expand their market presence and gain a competitive edge. For example, they are investing in research and development activities to develop new aptamer-based products with improved properties such as higher affinity, stability, and specificity.
In addition, strategic collaborations, partnerships, and mergers & acquisitions are also commonly seen in the aptamers market. These collaborations and partnerships allow companies to share their knowledge, expertise, and resources for the development of novel aptamer-based products. Moreover, mergers and acquisitions help companies to expand their product portfolio, increase their market share, and strengthen their position in the market.
Some of the major players operating in the global market include Base Pair Biotechnologies, SomaLogic, Aptadel Therapeutics, Aptamer Group, Noxxon Pharma, Aptagen, TriLink Biotechnologies, Altermune, Vivonics, and AM Biotechnologies.
Recent Developments
In January 2023, Aptamer Group collaborated with the BaseCure Therapeutics to develop optimer targeted therapies.
In April, 2020, Achiko signed a partnership agreement with Pengurus Wilayah Nahdlatul Ulama DKI for the marketing of aptamer based COVID kit “Aptamex”. Organization has around 90 Mn members in Indonesia.
Aptamers Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2024 |
USD 2.9 billion |
|
Revenue forecast in 2032 |
USD 16.33 billion |
|
CAGR |
24.10% from 2024 – 2032 |
|
Base year |
2023 |
|
Historical data |
2019 – 2022 |
|
Forecast period |
2024 – 2032 |
|
Quantitative units |
Revenue in USD million/billion and CAGR from 2024 to 2032 |
|
Segments covered |
By Type, By Application, By Region |
|
Regional scope |
North America, Europe, Asia Pacific, Latin America; Middle East & Africa |
|
Key companies |
Base Pair Biotechnologies, SomaLogic, Aptadel Therapeutics, Aptamer Group, Noxxon Pharma, Aptagen, TriLink Biotechnologies, Altermune, Vivonics, and AM Biotechnologies. |
FAQ's
The Aptamers Market report covering key are type, application, and region.
Aptamers Market Size Worth $ 16.33 Billion By 2032.
The global aptamers market expected to grow at a CAGR of 24.1% during the forecast period.
North America is Aptamers Market.
key driving factors in Aptamers Market are Rising number of the advantages of aptamers over antibodies.
